Non-human primate (NHP) studies often involve larger animals that require higher doses of adeno-associated virus (AAV) vectors. Low-quality AAV vectors can trigger immune responses or increase toxicity in animals. To mitigate these risks and improve the quality and reproducibility of NHP studies, PackGene offers high-quality NHP-grade AAV vectors with accurate measurement of virus quantity, genome integrity, empty capsid rate, and strict contamination control. By using PackGene's high-quality NHP-grade AAV vectors, researchers can lower the safety risks, enhance efficacy, and generate more reliable and reproducible results in their NHP studies.
Preclinical non-human primate (NHP) studies are an essential step in the development of adeno-associated virus (AAV) based gene therapies before they can be tested in human clinical trials. However, there are several challenges associated with conducting preclinical NHP studies for AAV-based gene therapy. NHP studies can be expensive to conduct due to the high cost of animal care, housing, and maintenance. Additionally, the cost of AAV vector production and characterization can also be significant. In addition, the use of NHPs in research is strictly regulated and requires a significant amount of paperwork and approvals, which can delay study timelines. The availability of NHPs can be limited, particularly for certain species or strains, which can make it difficult to conduct large-scale studies. NHPs can develop an immune response against the contaminants in AAV vector, which can affect the safety and efficacy of the therapy. This immune response can be difficult to predict, particularly in cases where the AAV vector is delivered systemically.
Conducting preclinical NHP studies for AAV-based gene therapy requires careful consideration of these challenges and a rigorous approach to study design, execution, and data analysis to ensure that the therapy is safe and effective for human use.
Providing high quality AAV is important for NHP studies because it directly impacts the safety, efficacy, and reproducibility of the study.
Firstly, AAV vectors can be immunogenic and provoke an immune response in the host, particularly in NHPs. This can complicate the interpretation of results, and can even impact the safety of the study, particularly in the case of systemic administration. High-quality AAV vectors with low levels of impurities and empty capsids can reduce the immune response to the therapy and improve the reproducibility of the results.
Secondly, AAV vectors with poor quality or purity can lead to variability between NHPs, making it challenging to draw accurate conclusions from small sample sizes. High-quality AAV vectors can improve the consistency of the study and reduce the variability between individual animals.
Thirdly, the accuracy and reproducibility of preclinical studies depend on the quality of the AAV vectors used. Any variability or uncertainty in the quality of the AAV vector can impact the results, and it can be challenging to attribute any observed effects to the therapy itself. By providing high-quality AAV vectors, researchers can ensure that any observed effects are due to the therapy itself and not confounded by issues related to vector quality.
High-quality AAV vectors is essential for preclinical NHP studies of AAV-based gene therapy. It ensures that the study is conducted with the highest level of rigor, reduces the risk of confounding factors impacting the results, and improves the accuracy, reproducibility, and translatability of the study.
Endotoxin and Contamination control
Precisely quantified Titer, Genome copy and integrity
Lower safety risks and enhance efficacy
Reproducible results
| AAV type | Scale | Turnaround time |
|---|---|---|
| Standard capsids (guaranteed yield) | 5E+13 GC~8E+15 GC | Start from 17 business days |
| Custom capsids(by volume) | Up to 100L with 30mL-200mL small scale test | Start from 25 business days |
The yield of AAV is dependent on various factors, such as serotypes, the gene of interest, and other variables. We offer a guaranteed package for commonly used AAV serotypes that are known to have high yields, such as 1, 3b, 5, 7, 8, 9, and Rh10. For other serotypes with low or unknown yields, we produce them based on volume. To determine the necessary production volume to achieve the target yield, we can conduct a small-scale test. If you’re unsure whether your serotypes qualify for our guaranteed package, please reach out to our technical support team for more information.
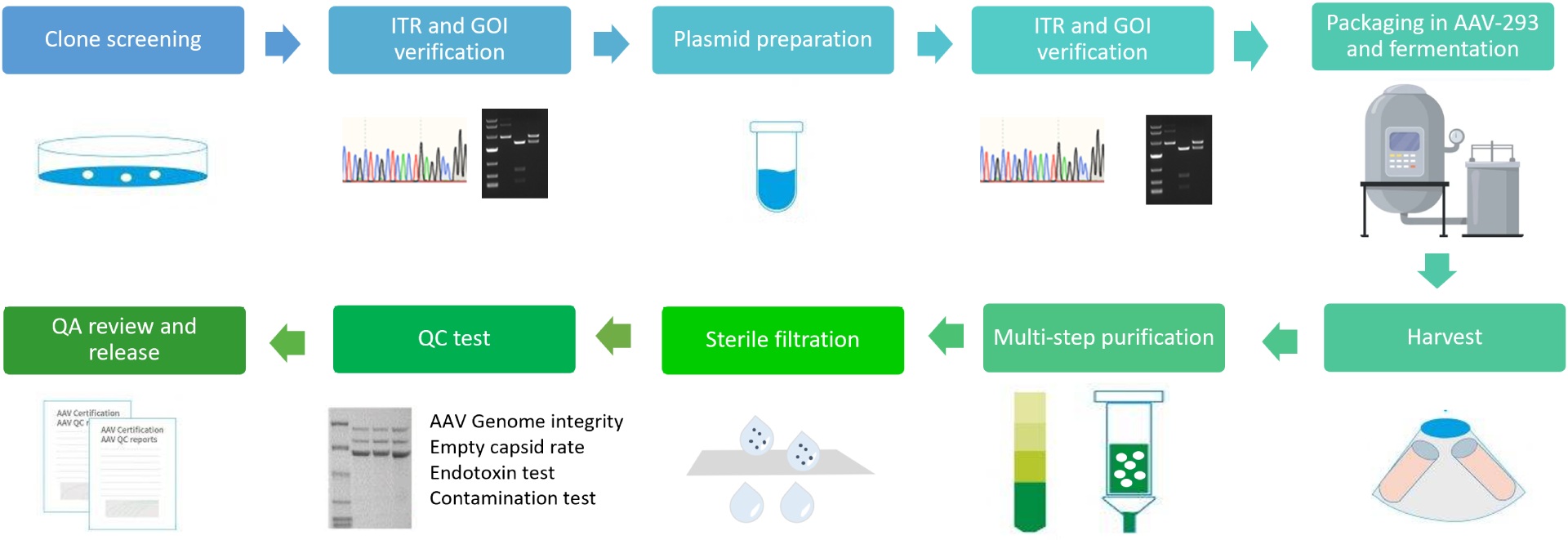
The AAV NHP (non-human primate) grade production process involves several steps to ensure the production of a high-quality and safe product. The process begins with clone screening and ITR (inverted terminal repeat) verification to ensure the correct vector sequence. Plasmid preparation and ITR and GOI (gene of interest) verification are then carried out to confirm the correct expression of the gene.
Next, co-transfection is performed in HEK 293T cells, which involves introducing the AAV cis-plasmid, Helper plasmid and Rep-Cap plasmid into the cells to produce the AAV vectors. Small scale culture may be conducted to determine the optimal conditions for growth and vector production. Following this, the cells are cultured or fermented to produce the AAV vectors.
The crude purification step separates the AAV vectors from cellular debris and other contaminants. Concentration and ultracentrifugation are then carried out to concentrate and further purify the AAV vectors. The sterile filtration step removes any remaining microorganisms.
The final step is a series of quality control (QC) tests, including AAV genome integrity, empty capsid rate, endotoxin tests, and contamination tests, etc. The results of these tests are reviewed by the quality assurance (QA) team, who then release the AAV vectors for use.
The benefits of this production process are that it minimizes safety risks, enhances efficacy, and ensures reproducible results, making it suitable for use in non-human primate studies.
| Test | Method | QC Standard | |
| Standard QC | ddPCR | Measure titer, normalized to 1e13vg/ml | Standard capsids: Concentration and total quantity meet needs. Custom capsids: quantity based on production scale. |
| AAV Capsid size* | SDS-PAGE silver stain | Match capsid protein size | |
| Guarantee endotoxin | LAL | <1EU/ml | |
| Mycoplasma Detection | qPCR | Negative | |
| Bioburden | Direct inoculation | No growth | |
| AAV Genome integrity | CE(titer>1e+12vg/ml, volume >50ul) | Report | |
| Empty Capsid Rate | TEM | <20% | |
| Additional QC | AAV Genome sequencing | Nanopore | Report |
| Empty Capsid Rate | AUC | Report | |
| Endotoxin removal | LAL | <0.2EU/ml | |
| Residual Triton Analysis(bundle with endo removal) | HPLC | 5ppm | |
| Sterility test | Direct inoculation | No growth |
NHP grade AAV undergoes stringent QC tests before being released to ensure the quality of the AAV product and minimize the adverse effects in animal studies.
Learn more about AAV NHP grade QC tests
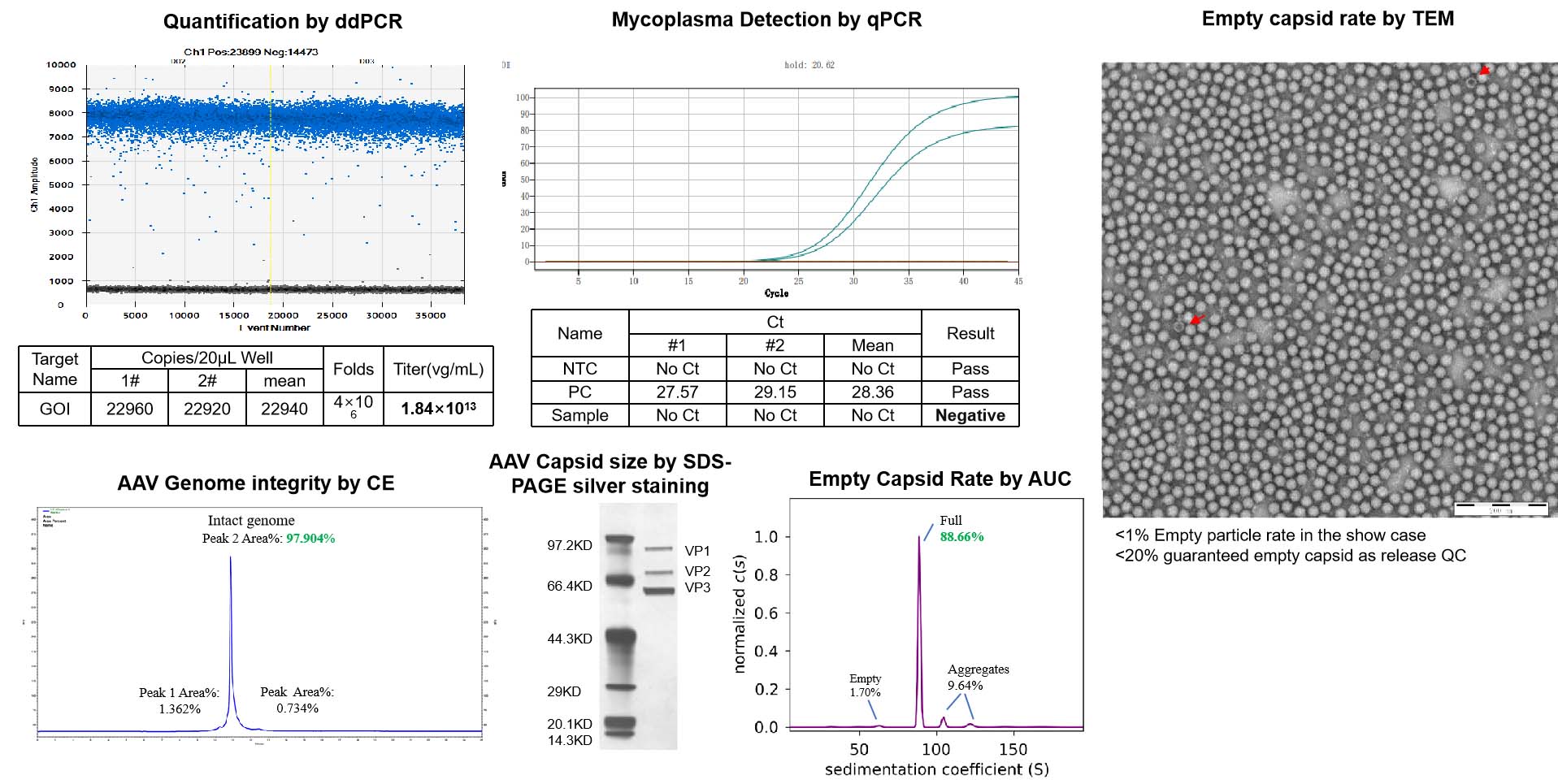
This test measures the titer of the AAV product and normalizes it to 1e13vg/ml. The concentration and total quantity of the product will meet the guaranteed yield package. For non-guaranteed yield, the quantity is based on the production scale.
This test uses TEM (transmission electron microscopy) to measure the percentage of empty capsids in the AAV product. The empty capsid rate must be below 20%.
This test uses LAL (limulus amebocyte lysate) to detect endotoxin levels, which must be below 1EU/ml.
This test uses qPCR to ensure that there is no mycoplasma contamination in the AAV product.
This test uses direct inoculation to detect the presence of any microbial growth in the AAV product.
This test uses CE (capillary electrophoresis) to detect the integrity of the AAV genome. The results of this test are reported.
This test uses SDS-PAGE silver stain to verify the capsid protein size of the AAV product
Plasmids used for AAV NHP Grade exceeds industrial standards
In addition to the basic standards, there are also advanced requirements that are not included in the default package and are available upon request with additional cost. These advanced requirements include residual host protein, advanced endotoxin removal, bioburden, mycoplasma contamination, ITR Nanopore sequencing, animal-free production, material archiving, pH (7.5~8.0), residual Kan, USP sterility, dedicated purification resin or filtration membrane, etc.
| Test | Method | QC Standard | |
| Basic standards | Appearance | Visual inspection | Clear, colorless, no visible particulates |
| A260:280 | Nanodrop | 1.8 – 2.0 | |
| Homogeneity | Agarose gel, supercoil >90±10% | Supercoil >90±10% | |
| Restriction Analysis | SmaI/AhdI | Conforming to reference pattern | |
| Residual RNA | Agarose gel | Not visible with 200ng load | |
| Residual E. coli DNA | Agarose gel | <15% of total band with 200ng load | |
| Endotoxin | LAL | ≤0.01 EU/µg | |
| ITR and GOI Sequencing | Sanger | 100% match ITR and GOI correct and clean peak | |
| Additional QC | Residual Host Protein | Nano orange | <0.1% by HCP ELISA |
| Advanced Endotoxin Removal <0.005 EU/µg |
Quantitative endotoxin test | <0.005 EU/µg | |
| Bioburden Testing | LB plate | No growth | |
| Mycoplasma Contamination | Quantitative PCR | Negative | |
| Competent cell | JM108, Sure, Sure2, Stbl3, NEBStbl | Customer define | |
| ITR integrity | Nanopore sequencing | Report | |
| Animal Free Production* | TSE/BSE | ||
| Material Archiving | Yes (plasmid+bacteria) | ||
| pH | 7.5~8.0 | ||
| Kan | ELISA | no detectable | |
| USP sterility | Yes | ||
| Dedicated purification resin | Upon request | ||
| Buffer | ddH2O, TE or customer defined | ||
| Dispensing | Upon request | ||
| Dedicated filtration membrane | Upon request |
Performance
- ddPCR (droplet digital PCR) is a highly sensitive method for quantifying the amount of AAV in a sample. It allows accurate and precise measurement of AAV genome copies per milliliter.
- qPCR (quantitative PCR) is a method for detecting and quantifying Mycoplasma contamination in a sample. Mycoplasma is a common contaminant in cell cultures and can affect AAV quality, so confirming its absence is important.
- Capillary Electrophoresis (CE) is a technique used to analyze the integrity of the AAV genome. CE can detect the presence of truncated or rearranged AAV genome fragments, which can affect AAV potency and safety.
- SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and silver stain are methods for analyzing the size and purity of the AAV capsid protein. These methods can detect the presence of impurities and ensure that the capsid size is consistent with the expected size for the AAV serotype.
- TEM (transmission electron microscopy) and AUC (analytical ultracentrifugation) are methods for determining the proportion of empty capsids in the AAV sample. Empty capsids are undesirable as they do not contain the therapeutic payload and can reduce the efficacy of the AAV vector. Monitoring the empty capsid rate is important to ensure the potency and quality of the AAV product.
The combination of these tests allows for a comprehensive assessment of the AAV quality, purity, and safety, ensuring that the AAV produced by PackGene is suitable for use in NHP studies.
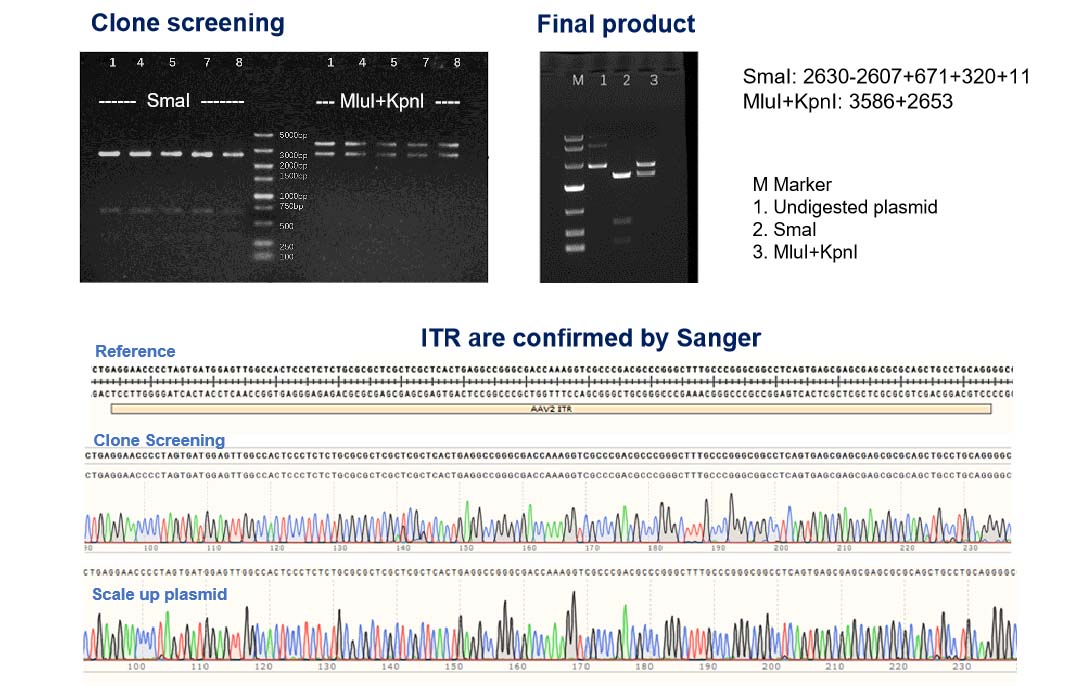
Upon receiving the AAV cis plasmid template from our customers, we conduct a double-check of the ITR integrity in addition to verifying the GOI size during clone screening before scaling up the plasmid. After the plasmid preparations are finished, another round of ITR and GOI sequencing is performed to ensure maximum fidelity. You can rest assured that the plasmid we used for NHP grade AAV are of the highest quality and integrity.

AAV Packaging Services
Ranging from pilot to industrial-scale AAV packaging for both in vitro and in vivo studies.
READ MORE

AAV Analytical Services
We are one of the few vendors that can do ALL in-house.
READ MORE

AAV Capsid Engineering
READ MORE
