1. Introduction
Cancer continues to be a major health concern despite progress in traditional treatments like surgery, chemotherapy, and radiotherapy. Gene therapy provides an innovative approach by introducing therapeutic genes to cancer cells, enabling targeted interventions with fewer side effects. Among viral vectors, adeno-associated viruses (AAVs) are increasingly recognized for their effectiveness in gene delivery due to their ability to transduce both dividing and non-dividing cells. This review explores the use of AAVs in cancer therapy, focusing on their targeting strategies and therapeutic applications.
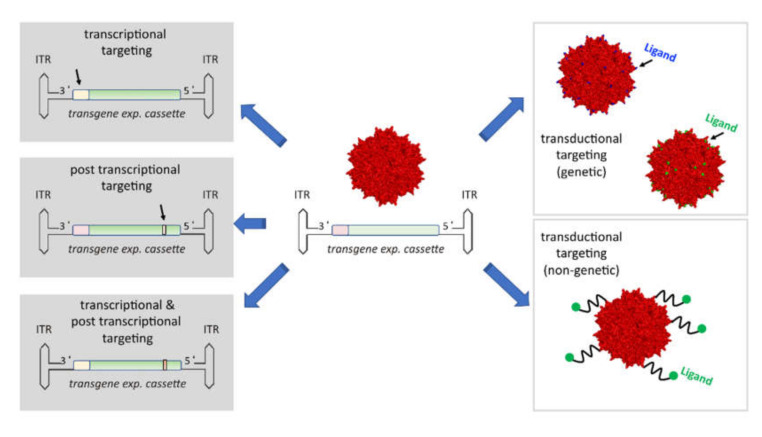
2. Targeting Strategies in AAV-Mediated Cancer Therapy
Achieving precise gene delivery to cancer cells or the tumor microenvironment is crucial to avoid off-target effects and ensure therapeutic efficacy. Several targeting strategies have been developed, including:
2.1 Transductional Targeting
- Capsid Modifications: Engineering AAV capsids with ligands or receptor-binding molecules enhances cell-specific uptake. For example, adding Her2-targeting ligands via AAV capsid engineering improves specificity for cancer cells expressing the Her2 receptor.
- Biodistribution Control: Modifying capsid proteins can limit off-target transduction and reduce immune responses by preventing uptake in healthy tissues. This is particularly important when using AAV CRISPR vectors for precise gene editing.
2.2 Transcriptional Targeting
- Promoter Design: Incorporating tissue- or tumor-specific promoters ensures that therapeutic genes are only expressed in targeted tissues, minimizing the risk of adverse effects in healthy cells. This is crucial to generate safe therapeutic vectors prior to scale up for AAV production.
2.3 Post-Transcriptional Targeting
- MicroRNA (miRNA) Regulation: By inserting miRNA target sites into the AAV genome, therapeutic transgene expression can be suppressed in healthy tissues where specific miRNAs are active. It prevents unnecessary damage to non-cancerous cells such as hepatocytes. This technique can be optimized using AAV-miRNA cloning to screen a group of candidate miRNA for enhanced specificity
3. Applications of AAV Vectors in Cancer Therapy
AAV vectors offer several gene therapy applications, including suicide gene therapy, RNA interference (RNAi), and anti-angiogenesis therapy.
3.1 Suicide Gene Therapy
- Mechanism: This approach introduces genes that convert non-toxic prodrugs into cytotoxic compounds.
- Example: An AAV system expressing herpes simplex virus thymidine kinase (HSV-tk) activates ganciclovir (GCV), leading to tumor cell death. This strategy has shown promise in head and neck cancer, bile duct cancer, and bladder carcinoma models.
3.2 RNA Interference (RNAi) Therapy
- Mechanism: AAV vectors are ideal for delivering small RNA molecules, such as shRNA, that silence genes critical for cancer progression. Efficient AAV shRNA cloning can enhance this therapy’s effectiveness.
- Example: In a prostate cancer model, AAV-mediated knockdown of the androgen receptor gene reduced tumor growth. In hepatocellular carcinoma models, RNAi therapy induced apoptosis without toxicity to healthy cells.
3.3 Anti-Angiogenesis Therapy
- Mechanism: AAV vectors inhibit tumor growth by disrupting angiogenesis, the formation of new blood vessels that supply tumors with nutrients. A well designed AAV vector including optimized promotor and anti-angiogenic genes like VEGF traps and PEDF shows promising results.
- Examples:
① VEGF Trap Protein: AAV vectors expressing this high-affinity receptor suppressed breast cancer growth and prevented lung metastases.
② Pigment Epithelium-Derived Factor (PEDF): PEDF delivery via AAV inhibited metastasis and extended survival in colorectal carcinoma models.
③ Kallistatin: This anti-angiogenesis factor, when delivered by AAV, triggered apoptosis in hepatocellular carcinoma models.
3.4 Cancer Immunotherapy
- Mechanism: AAV vectors are advancing cancer immunotherapy by modifying immune cells, such as CAR-T cells, providing a safer alternative to integrating viral vectors. AAV CRISPR vectors enable precise CAR generation, targeting specific loci like TRAC to enhance anti-tumor responses. AAV Cas9 systems also support precise gene editing in immune cells. Additionally, AAV vector construction and packaging allow for the delivery of cancer vaccines and immunomodulating cytokines like IL-12 and IL-27, reshaping the tumor microenvironment to boost immune responses.
- Example: Studies have shown that AAV CRISPR vectors can efficiently insert CARs into the TRAC locus of T cells, achieving over 40% CAR insertion and improved anti-tumor potency. AAV vector-mediated delivery of IL-27 has been used to reduce tumor growth and improve immune cell infiltration in various tumor models. High-yield AAV particle production services are supporting these emerging therapeutic applications by providing scalable solutions for AAV vector production and packaging.
4. Challenges and Limitations
Although preclinical studies have shown encouraging results, several challenges remain for AAV-based cancer therapies:
- Long-Term Transgene Expression: Prolonged gene expression may lead to complications if the tumor recurs or if therapy becomes ineffective.
- Resistance and Tumor Escape Mechanisms: Recurrent tumors may develop resistance, similar to chemotherapy.
- Capsid Engineering Complexity: Developing customized capsids is time-consuming, but screening capsid libraries offers a promising alternative.
- Immune Response: Neutralizing antibodies against AAV can limit vector re-administration, reducing therapeutic efficacy.
5. Current Progress and Future Directions
Although human trials using AAV vectors for cancer therapy are still limited, promising results are emerging from preclinical models:
- Clinical Trial Example: An ex vivo AAV-based gene therapy trial targeting gastric cancer is currently active (NCT02496273).
- Proof-of-Concept Study: AAV-mediated interferon β (IFNβ) expression suppressed tumor growth in colorectal cancer models and improved survival in glioblastoma patients when combined with temozolomide therapy.
- Capsid Screening: AAV7 and AAV9 vectors demonstrated high transduction efficiency in prostate tissue, showing potential for targeted miRNA knockdown in prostate cancer models.
Future research must focus on regulated gene expression strategies and improved capsid designs to address safety and efficacy concerns. Understanding tumor biology will also be essential to develop more effective therapies.
In addition, efforts to address safety concerns and scale up AAV high-yield production for clinical use are essential for accelerating research and development and are being actively explored.
6. Conclusion
AAV vectors represent a versatile platform for cancer gene therapy, capable of delivering a variety of therapeutic agents such as shRNA, CRISPR-Cas9, anti-angiogenic factors, and immunotherapy approaches. AAV-based therapies represent a promising frontier in cancer treatment by enabling targeted gene delivery and expression within tumors. While challenges remain, such as immune responses and resistance, ongoing research and clinical trials will likely refine these strategies. With advances in AAV vector design, packaging and production, capsid engineering, transcriptional control, and tumor-specific targeting, AAV vectors could become valuable tools in personalized cancer therapy.
1.https://pmc.ncbi.nlm.nih.gov/articles/PMC7409174/
2.https://pmc.ncbi.nlm.nih.gov/articles/PMC10000783/
3.https://pmc.ncbi.nlm.nih.gov/articles/PMC10000783/
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
[2024/12/27] Gene and Cell Therapy- weekly digest from PackGene
FeaturedNewsArticlesPackGene's NewsletterReceive the latest news and insights to your inbox.About PackGenePackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span...
GEMMABio Pioneers Affordable Gene Therapies with $34M Seed Funding
Gene therapy startup GEMMABio, led by industry trailblazer Jim Wilson, has secured $34 million in seed funding to transform treatment options for rare diseases. Wilson, formerly of the University of Pennsylvania, founded GEMMABio and Franklin Biolabs earlier this...
Genethon Highlights Key Developments in Gene Therapy Research
December 19, 2024 – Paris, France – Genethon, a pioneering non-profit gene therapy research and development organization founded by the French Muscular Dystrophy Association (AFM-Telethon), has unveiled significant progress in its clinical and scientific endeavors as...
Sumitomo Pharma America Announces U.S. FDA Approval of GEMTESA® (vibegron) for Men with Overactive Bladder Symptoms Receiving Pharmacological Therapy for Benign Prostatic Hyperplasia
– GEMTESA® is the first and only beta-3 agonist approved for the treatment of men with OAB symptoms who are receiving pharmacological therapy for BPH – MARLBOROUGH, Mass., Dec. 23, 2024 /PRNewswire/ -- Sumitomo Pharma America, Inc. (SMPA) announced today that the U.S....
Related Services
AAV Packaging Services
READ MORE

