
HD is an autosomal dominant genetic disorder with a global prevalence of approximately 2.7/100,000 and an incidence rate of about 0.38/100,000 annually. The prevalence is around 5.7/100,000 in Europe, North America, and Australia, and about 0.4/100,000 in Asia. The causative gene for HD is the huntingtin gene (HTT), which encodes a large protein with over 3,000 amino acids. An abnormal expansion of CAG trinucleotide repeats in the first exon of HTT leads to misfolding of the huntingtin protein (mHTT). This mutated protein accumulates in the brain, ultimately causing neurological dysfunction and degeneration. Targeting the suppression of mHTT expression may be key to treating Huntington’s disease.
On May 17, 2024, Professors Xiaojiang Li and Su Yang from Jinan University published their latest research findings in the journal Science Advances. Their study reported previously unidentified pathogenic mechanisms of HTT and discovered a primate-specific E3 ubiquitin ligase, TRIM37. TRIM37 promotes the ubiquitination and degradation of mutant HTT (mHTT) and regulates its aggregation in the brains of mice and monkeys. Therefore, TRIM37 protein can be considered a potential therapeutic target for Huntington’s disease by modulating mHTT expression. PackGene provided AAV packaging services for this study.
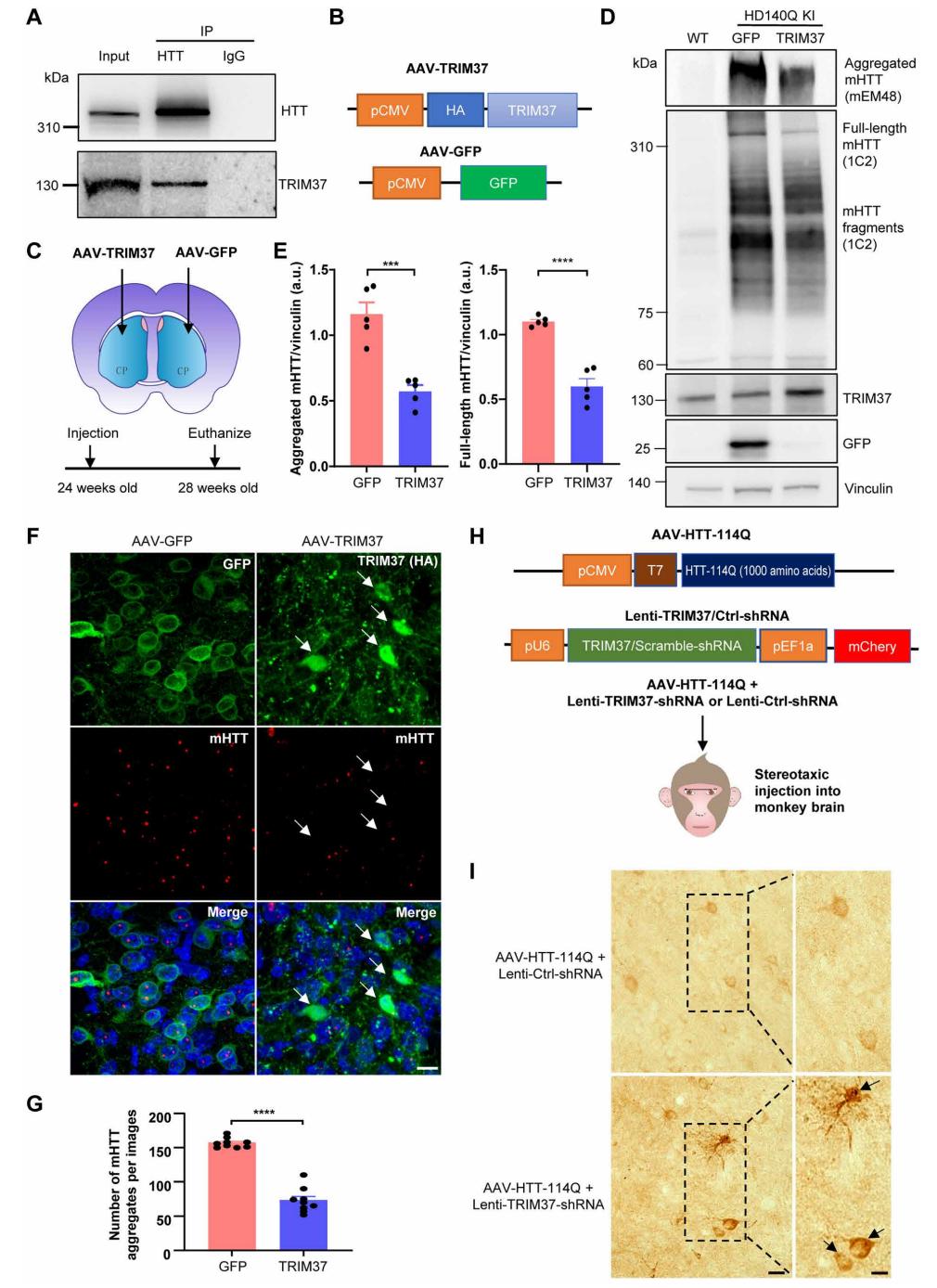
https://pubmed.ncbi.nlm.nih.gov/38758800/
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
More Articles
Advancing AAV-Based Gene Therapy for Hearing Loss Using Mini-PCDH15 Variants
Hearing loss affects millions of people worldwide and can be caused by genetic defects in key proteins essential for auditory function. Recent research by Pedro De-la-Torre and colleagues (doi: https://doi.org/10.1101/2024.06.16.599132) has provided significant...
AAV Vectors in Cancer Therapy: A Review of Applications and Strategies
1. Introduction Cancer continues to be a major health concern despite progress in traditional treatments like surgery, chemotherapy, and radiotherapy. Gene therapy provides an innovative approach by introducing therapeutic genes to cancer cells, enabling targeted...
Advances in AAV-SB Transposon Hybrid Systems for Liver-Targeted Gene Therapies
*Nicolás Sandoval-Villegas, Zoltán Ivics, The best of both worlds: AAV-mediated gene transfer empowered by LNP delivery of Sleeping Beauty transposase for durable transgene expression in vivo, Molecular Therapy, Volume 32, Issue 10, 2024, Pages 3211-3214, ISSN...
Novel Approach in T Cell Engineering: Lipid Nanoparticles Enable Advanced Genome Editing for Cancer Therapies
Revolutionizing CAR T Cell Therapy with Lipid Nanoparticles Chimeric antigen receptor (CAR) T cell therapy has transformed cancer treatment by turning a patient’s own T cells into powerful cancer-fighting agents. However, as the technology advances, there is an...
Related Services
AAV Packaging Services
READ MORE
AAV Packaging Service (NHP)
READ MORE
AAV Packaging Service (HT)
READ MORE

