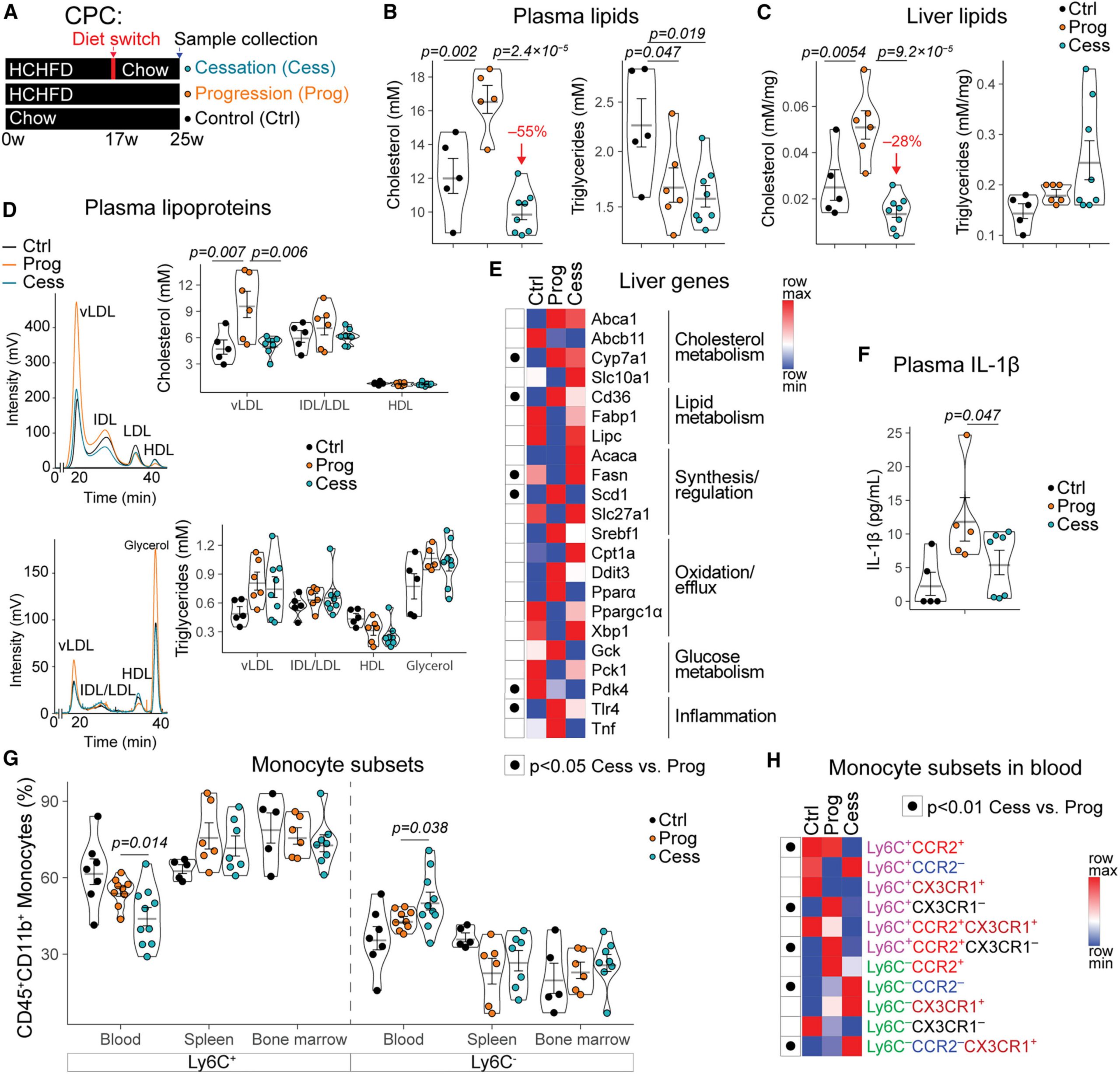
Systemic metrics of atherosclerosis resolution in dietary cessation CPC model. Credit: Cell Reports (2024). DOI: 10.1016/j.celrep.2024.114911
Researchers at Case Western Reserve University have identified a new target to treat atherosclerosis, a condition where plaque clogs arteries and causes major cardiac issues, including stroke and heart attack.
In a new study, published in the journal Cell Reports, the team identified an inflammation-reducing molecule—called itaconate (ITA)—that could be the foundation of a new approach to treat such a common and deadly disease.
Heart disease is the leading cause of death for men, women and people of most racial and ethnic groups, according to the U.S. Centers for Disease Control and Prevention.
Medications help but don’t completely protect patients from cardiovascular risk. So, doctors also recommend lifestyle changes, such as a low-cholesterol/low-fat diet (LCLFD), to further reduce plaque and inflammation that increase the risk of cardiovascular disease. Yet many patients find it challenging to follow diet restrictions long-term.
Identifying the role ITA plays in diet and heart disease may help address this.
“We’ve found that itaconate is crucial to the diet’s ability to stabilize plaques and reduce inflammation, which has been a mystery until now,” said Andrei Maiseyeu, associate professor at the Cardiovascular Research Institute and Department of Biomedical Engineering at Case Western Reserve’s School of Medicine.
“This discovery marks a major leap forward in the understanding of how diet-induced plaque resolution occurs at a molecular level.”
Based on their discovery, Maiseyeu and his team have developed a new treatment: ITA-conjugated lipid nanoparticles. This new therapeutic approach allows ITA to accumulate in plaque and bone marrow, where it reduces inflammation and mimics the beneficial effects of LCLFD without requiring drastic lifestyle changes.
“We have already seen its effectiveness in multiple models of atherosclerosis,” Maiseyeu said. “We are optimistic that this will result in better treatments that will greatly lower the long-term risk of heart attacks and strokes while also improving patients’ quality of life.”
Maiseyeu and his team are now taking steps to translate ITA-LNP to the clinic, including engineering a pill form of the treatment, which they believe will not only be convenient for patients, but also transformative.

Check out our AAV CDMO service to expedite your gene therapy research
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
[2024/12/20] Gene and Cell Therapy- weekly digest from PackGene
FeaturedNewsArticlesPackGene's NewsletterReceive the latest news and insights to your inbox.About PackGenePackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span...
Sangamo and Astellas Collaborate to Advance Neurological Gene Therapies Using AAV Capsid Technology
Sangamo Therapeutics, Inc. (Nasdaq: SGMO), a leader in genomic medicine, and Astellas Pharma Inc. (TSE: 4503), a global innovator in life sciences, have partnered under a new license agreement. This collaboration centers around Sangamo’s cutting-edge neurotropic AAV...
Inceptor Bio and GRIT Bio Announce Strategic Partnership to Advance IB-T101, a Next-Generation Solid Tumor CAR-T Utilizing the OUTLAST™ Platform
SHANGHAI and MORRISVILLE, N.C., Dec. 18, 2024 /PRNewswire/ -- Inceptor Bio, a leading innovator in cell therapy, and GRIT Bio, a clinical-stage immunotherapy developer, today announced a strategic partnership to advance IB-T101, a potentially best-in-class CAR-T...
Proof-of-concept study bioengineers therapeutics for improved cancer treatment
Credit: Pixabay/CC0 Public DomainA team of Children's Medical Research Institute (CMRI) scientists has identified a new method for producing a therapeutic product that has the potential to improve the treatment of cancer. The work by Associate Professor Leszek...
Related Services

AAV Packaging Services
READ MORE

Off-the-Shelf AAV Products
READ MORE

