An international team led by Goethe University Frankfurt has identified an intracellular sensor that monitors the quality of so-called MHC-I molecules, which help the immune system recognize and kill harmful cells, including tumor cells. The sensor ensures that defective MHC-I molecules remain inside the cell, where they are eventually degraded. Surprisingly, a lack of this quality assurance can lead to more MHC-I molecules reaching the surface of cancer cells, triggering a stronger immune response against the tumor.
The findings are published in the journal Cell.
It is comparatively easy to tell a cell’s state of health: On their surface, cells present fragments of almost all the proteins they contain inside. This means the immune system can directly recognize whether a cell has been infected by a virus or has been dangerously altered by a mutation.
Countless molecular “radio masts”—the MHC-I molecules—are responsible for presenting these fragments. They are assembled inside the cell and then transported to the membrane, the lipid layer surrounding the cell.
Here, the masts are anchored such that the cargo faces outside and can be detected by troops of the immune system constantly patrolling the body. If these troops detect harmful molecules being presented on the MHC-I radio masts, they kill the relevant cell. A requirement, however, is that the masts themselves are fully functional; otherwise, there is a risk that this mechanism will not work and harmful cells escape the immune system.
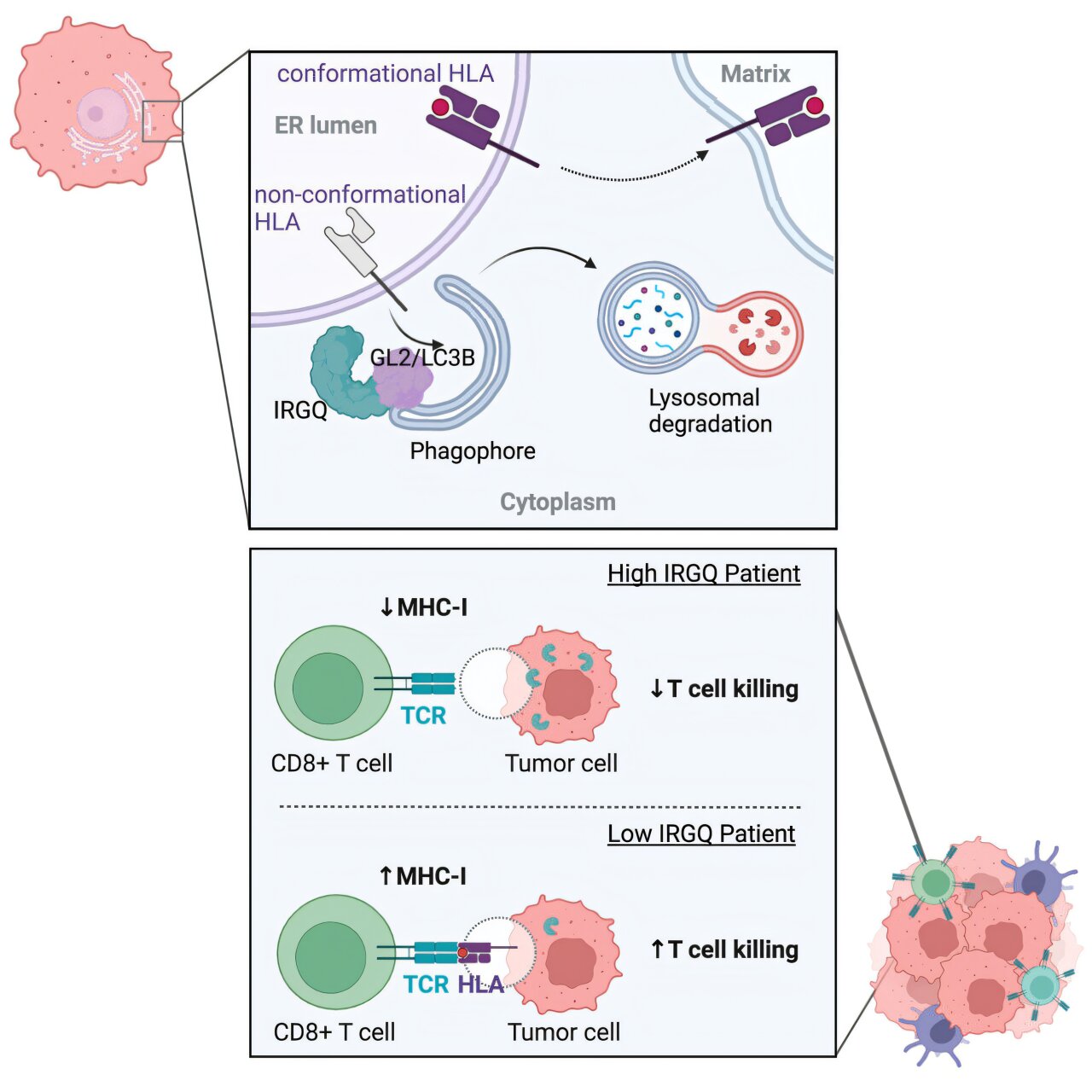
“We have now discovered a sensor inside the cell that ensures that only functional MHC-I molecules are transported to the plasma membrane, while the defective units are eliminated,” explains Dr. Lina Herhaus, who until recently conducted research on this topic at Goethe University’s Institute of Biochemistry II and is now leading an independent research group at Braunschweig-based Helmholtz Centre for Infection Research.
Cells continuously produce a large number of proteins to sustain their manifold functions. If errors occur during this process, the affected molecules are usually eliminated. Specialized receptors recognize defective proteins and route them to mini garbage bags, where they are broken down.
“As part of our study, we searched for yet unknown receptors and came across a protein called IRGQ, which is specifically responsible for ensuring the quality control of the MHC-I radio masts,” says Herhaus.
The researchers used genetic interference to suppress the production of IRGQ. The result: defective radio masts accumulated in the cells, some of which were also incorporated into the cell membrane, together with their functional counterparts.
“You would actually expect cells without IRGQ to trigger a weaker immune response. However, this is obviously not the case: Having analyzed various human tumors, we found that less IRGQ was associated with a better survival rate of patients with liver cancer,” explains Prof. Ivan Đikić from the Institute of Biochemistry II, who co-led the study with Herhaus.
The patient data was also confirmed in an experimental liver cancer mouse model: In animals without IRGQ, the immune system attacked tumor cells much more aggressively; as a result, the rodents without IRGQ survived the cancer significantly longer.
IRGQ could represent a target structure for new drugs, at least for liver cell carcinomas—the world’s second most deadly type of cancer. “We have found a new mechanism by which tumor cells evade the immune system. In further studies, we will now examine IRGQ’s influence on other types of cancer,” emphasizes Đikić.
“Our findings could be used in future to develop new therapies for liver cancer. One example would be to use drugs to target IRGQ for degradation and thereby stimulate the immune response against the cancer.”
Irrespective of this, the newly discovered mechanism is also exciting for basic research. “We want to find out how important IRGQ is for the functioning of the immune system in general, including during viral infections,” says Herhaus. “The results of our study raise a whole series of interesting questions, the answers to which can deepen our understanding of the body’s immune defense.”

Check out our AAV CDMO service to expedite your gene therapy research
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
[2024/11/8] Gene and Cell Therapy- weekly digest from PackGene
FeaturedNewsArticlesPackGene's NewsletterReceive the latest news and insights to your inbox.About PackGenePackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span...
Gene Therapy Discovery: Stem Cells Tailor Their Behavior to Specific Genetic Diseases
Researchers at the RCCS San Raffaele Scientific Institute in Milan have uncovered that hematopoietic stem cells (HSCs) adapt their lineage commitment during gene therapy based on the specific genetic disease being treated, providing critical insights for advancing...
Hospital-Based CGT Production the Best Option for Patients
Manufacturing cell and gene therapies at hospitals in dedicated, GMP-accredited facilities would overcome many of the technical and logistical challenges associated with decentralized production. So says Na Kyung Lee, PhD, from Sungkyunkwan University in Seoul, Korea,...
FDA Grants First Clearance for CRISPR/Cas13 RNA-Editing Therapy HG202 for Macular Degeneration
SHANGHAI and CLINTON (NJ), November 4, 2024 – HuidaGene Therapeutics, a global clinical-stage biotechnology company focused on genome medicines, has achieved a groundbreaking milestone. The US FDA has cleared its investigational new drug (IND) application for HG202,...
Related Services

AAV Packaging Services
READ MORE

Off-the-Shelf AAV Products
READ MORE

