The scientists recently described their low-toxicity carriers in ACS Nano, in an article titled, “Widespread Gene Editing in the Brain via In Utero Delivery of mRNA Using Acid-Degradable Lipid Nanoparticles.” According to this article, “densely PEGylated lipid nanoparticles (ADP-LNPs) containing an acid-degradable PEG–lipid can safely and effectively deliver mRNA for gene editing enzymes to the fetal mouse brain, resulting in successful transfection and editing of brain cells.”
When the scientists tested their LNP technology, they used a mouse model of Angelman syndrome. Encouraged by the technology’s performance, they speculated that their LNP technology could be effective not just against Angelman syndrome, but against other genetic-based neurodevelopmental conditions, such as Rett syndrome and Hurler syndrome.
“The implications of this tool for treating neurodevelopmental conditions are profound,” said Aijun Wang, PhD, one of the study’s senior authors and a UC Davis professor of surgery and biomedical engineering. “We can potentially correct genetic anomalies at a foundational level during critical periods of brain development.”
The new LNP technology is the result of a collaboration between UC Davis scientists led by Wang and UC Berkeley scientists led by Niren Murthy, PhD, another senior author of the current study and a professor of bioengineering. The team hopes to develop this technology into treatments for genetic conditions that can be diagnosed during prenatal testing. Such treatments could be given in the womb to avoid more damage as cells develop and mature.
Previously, Wang, Murthy, and colleagues reported that they had engineered rapidly hydrolysable LNPs (RD-LNPs). In a Nature Nanotechnology paper titled, “Acid-degradable lipid nanoparticles enhance the delivery of mRNA,” they wrote, “Acid-degradable lipids composed of polyethylene glycol lipids, anionic lipids, and cationic lipids were synthesized with the azido-acetal linker and used to generate RD-LNPs, which significantly improved the performance of LNP-mRNA complexes in vitro and in vivo. Collectively, RD-LNPs delivered mRNA more efficiently to the liver, lung, spleen, and brains of mice and to hematopoietic stem and progenitor cells in vitro than conventional LNPs.”
In the current study, Wang, Murthy, and colleagues deployed their LNP-mRNA technology via in utero intracerebroventricular (ICV) injection. “In utero ICV injections of ADP-LNPs resulted in the transfection of [neural stem and progenitor cells], which proliferated and populated the entire brain by the time mice were fully developed,” the scientists wrote. “ADP-LNPs were also well tolerated after an in utero ICV injection, presumably because of their combination of dense PEGylation and acid degradability.”
“The treatment of neurodevelopmental disorders with gene editing therapeutics requires the development of delivery vectors that can transfect the brain tissue globally,” they continued. “In utero ICV injection with ADP-LNPs is so far the only methodology available for nonvirally transfecting large volumes of the brain tissue and is a promising platform for developing treatments for neurodevelopmental disorders.”
The researchers used their LNP technology to deliver guide mRNA and Cas9 mRNA. Using tracers, the researchers could see all the neurons that were edited inside the brain. Their study showed that the nanoparticles were taken up by the brain’s developing neural stem and progenitor cells. The nanoparticles led to gene edits in 30% of the brain stem cells in the mouse model.
“Transfecting 30% of the whole brain, especially the stem cells, is a big deal,” Wang asserted. “These cells migrate and spread to many places across the brain as the fetus further develops.”
In the study, as the fetal development continued, the stem cells proliferated and migrated to form the central nervous system. The study revealed that more than 60% of the neurons in the hippocampus and 40% of neurons in the cortex were transfected.
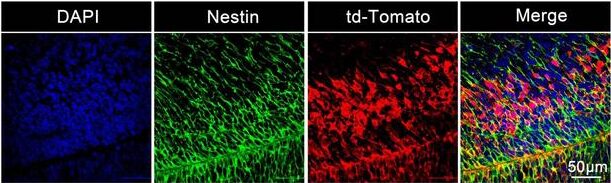
“This is a very promising method for genetic conditions affecting the central nervous system,” Wang explained. “When the babies are born, many of the neurons could have been corrected. This means the baby could be born with no symptoms.”
Wang expects to see an even higher percentage of transfected cells in a diseased mouse model: “Bad neurons with mutation may be killed by the accumulation of disease symptoms and good neurons may stay and proliferate. This could lead to amplified therapeutic efficiency. If we know well enough how cells work, we can leverage this knowledge to cooperate with the naturally occurring pathways in the cell.”

Check out our mRNA service to expedite your vaccine research
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
[2024/12/20] Gene and Cell Therapy- weekly digest from PackGene
FeaturedNewsArticlesPackGene's NewsletterReceive the latest news and insights to your inbox.About PackGenePackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span...
Sangamo and Astellas Collaborate to Advance Neurological Gene Therapies Using AAV Capsid Technology
Sangamo Therapeutics, Inc. (Nasdaq: SGMO), a leader in genomic medicine, and Astellas Pharma Inc. (TSE: 4503), a global innovator in life sciences, have partnered under a new license agreement. This collaboration centers around Sangamo’s cutting-edge neurotropic AAV...
Inceptor Bio and GRIT Bio Announce Strategic Partnership to Advance IB-T101, a Next-Generation Solid Tumor CAR-T Utilizing the OUTLAST™ Platform
SHANGHAI and MORRISVILLE, N.C., Dec. 18, 2024 /PRNewswire/ -- Inceptor Bio, a leading innovator in cell therapy, and GRIT Bio, a clinical-stage immunotherapy developer, today announced a strategic partnership to advance IB-T101, a potentially best-in-class CAR-T...
Proof-of-concept study bioengineers therapeutics for improved cancer treatment
Credit: Pixabay/CC0 Public DomainA team of Children's Medical Research Institute (CMRI) scientists has identified a new method for producing a therapeutic product that has the potential to improve the treatment of cancer. The work by Associate Professor Leszek...
Related Services

AAV Packaging Services
READ MORE

Off-the-Shelf AAV Products
READ MORE

