Researchers from the Yong Loo Lin School of Medicine, National University of Singapore (NUS Medicine) are working on a therapy that holds potential in treating patients with epilepsy, a neurological disorder defined by recurring seizures due to abnormal brain activity.
Led by Research Assistant Professor Huang Hua from the Department of Physiology and Electrophysiology Core Facility at NUS Medicine, they have trialed a novel gene therapy approach for a rare genetic form of epilepsy linked to a mutation in the KCNA2 gene in the human brain, which is associated with recurring seizures.
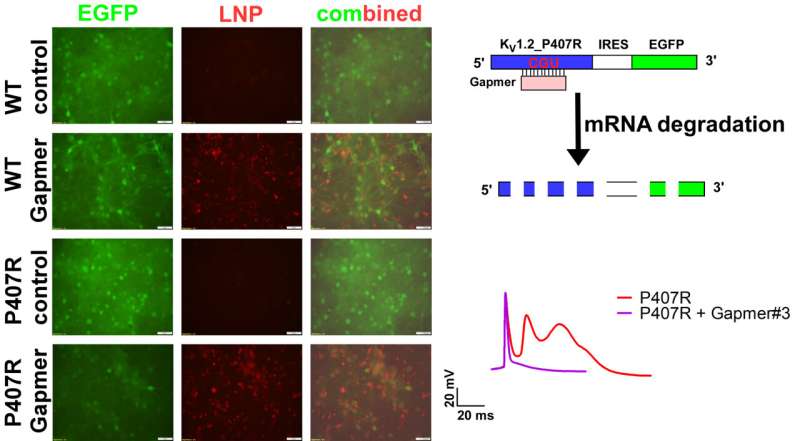
A specialized treatment called a Gapmer antisense oligonucleotide (ASO) is designed to specifically target and break down faulty ribonucleic acids (RNA) while keeping normal gene function intact. Using this RNA therapy led to a notable decrease in a problematic potassium channel protein encoded in the KCNA2 gene, which helped restore normal potassium flow and reduce excessive neuron activity linked to epilepsy.
Asst Prof Huang said, “Epilepsy is associated with hyper-excitable neurons, and potassium helps dampen the excitability levels. The potassium channel encoded by KCNA2 is like a door that controls the potassium ion flow on the surface of the cells—when the gene is mutated, the door fails to work and potassium cannot be released to control neuron activity, which results in epilepsy.
“Our therapy targets the faulty RNA in the gene and ‘fixes the door,’ so that potassium can flow and regulate the neuron activity levels.”
Published in Molecular Therapy—Nucleic Acids, the research study was conducted on in vitro cell samples. The research work began in 2021, when the team was approached by the family of an infant who suffered from multiple generalized seizures that were resistant to multiple medications and conventional treatments.
While the research work is in early stages and will need to undergo further testing in laboratory models before moving to clinical trials, the remarkable results from the research offer hope that the therapy can be delivered to patients suffering from severe epilepsy caused by channelopathies—genetic disorders caused by abnormalities in the ion channels of cells—within the next 10 to 20 years.
The new Gapmer technology being worked on by the research team could also be adapted to target other mutations in the same gene or other ion channel genes—opening the possibility of creating personalized treatments for different KCNA2-related issues, potentially offering hopeful outcomes for patients with rare forms of epilepsy that are unresponsive to standard medications.
Professor Soong Tuck Wah from the Department of Physiology and Electrophysiology Core Facility at NUS Medicine, a co-author of the study, said, “Our research seeks not only to address the unique challenges posed by this specific mutation, but also stems from our team’s desire to improve the quality of life for patients.
“Since the therapy has shown promise in targeting a specific gene mutation causing epilepsy, we hope to eventually pioneer new treatment options for patients suffering from this condition, and other similar gene mutations.”

Check out our AAV CDMO service to expedite your gene therapy research
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
[2024/12/20] Gene and Cell Therapy- weekly digest from PackGene
FeaturedNewsArticlesPackGene's NewsletterReceive the latest news and insights to your inbox.About PackGenePackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span...
Sangamo and Astellas Collaborate to Advance Neurological Gene Therapies Using AAV Capsid Technology
Sangamo Therapeutics, Inc. (Nasdaq: SGMO), a leader in genomic medicine, and Astellas Pharma Inc. (TSE: 4503), a global innovator in life sciences, have partnered under a new license agreement. This collaboration centers around Sangamo’s cutting-edge neurotropic AAV...
Inceptor Bio and GRIT Bio Announce Strategic Partnership to Advance IB-T101, a Next-Generation Solid Tumor CAR-T Utilizing the OUTLAST™ Platform
SHANGHAI and MORRISVILLE, N.C., Dec. 18, 2024 /PRNewswire/ -- Inceptor Bio, a leading innovator in cell therapy, and GRIT Bio, a clinical-stage immunotherapy developer, today announced a strategic partnership to advance IB-T101, a potentially best-in-class CAR-T...
Proof-of-concept study bioengineers therapeutics for improved cancer treatment
Credit: Pixabay/CC0 Public DomainA team of Children's Medical Research Institute (CMRI) scientists has identified a new method for producing a therapeutic product that has the potential to improve the treatment of cancer. The work by Associate Professor Leszek...
Related Services

AAV Packaging Services
READ MORE

Off-the-Shelf AAV Products
READ MORE

