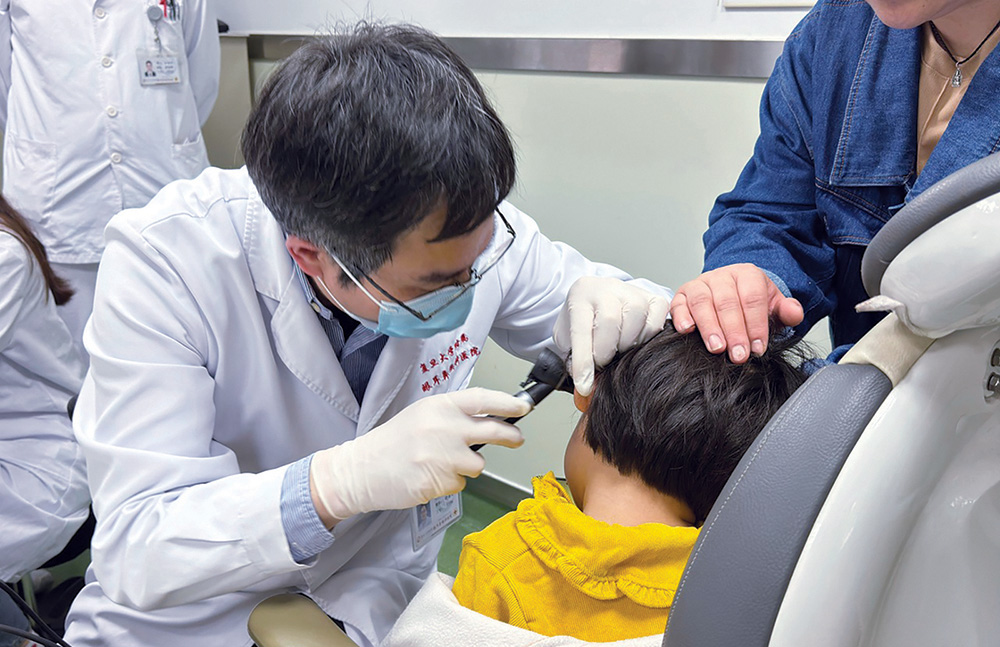
A hearing test for a newborn baby is routine. And if it reveals a hearing problem, the choice that is presented to those hoping to improve the baby’s hearing is also routine. There are usually two options: a cochlear implant or a hearing aid. But thanks to the efforts of many researchers, a third option is emerging: gene therapy. Over the past year, several advances in gene therapy for inherited hearing loss have brought a wave of excitement and momentum to the field.
In January, positive results were reported in two pioneering human trials. The larger of the two studies showed hearing recovery and improvements in speech recognition in five out of six children with DFNB9 (a form of autosomal recessive deafness caused by mutations in OTOF, the otoferlin gene). The work was published in The Lancet, in a paper titled, “AAV1-hOTOF gene therapy for autosomal recessive deafness 9: a single-arm trial.” It described how researchers used an adeno-associated virus (AAV) to deliver the human OTOF gene to the inner ears of patients.
One of the paper’s senior authors, Zheng-Yi Chen, DPhil, associate professor of otolaryngology–head and neck surgery, Harvard Medical School and Mass Eye and Ear, said that the study’s results were beyond his “wildest imagination.” Chen offered GEN an update on the trial, noting that the children are “doing really well” and that the hearing improvements have been sustained. The researchers are continuing to dose patients, both children and older patients, to define the time window for which the treatment works. More recently, the same group published another advance—this time in the administration of the gene therapy to both ears of children.

Chen has studied hearing for decades. He cloned one of the first deafness genes during his graduate work and has thought about developing a treatment ever since. Since then, advances like the human genome project and the development of mouse models have moved the field forward. But despite advances in basic research and the introduction of new tools, the lack of translational progress remained, in Chen’s view, a “glaring gap.” He remarked that for a long time, “We didn’t really know how to go about it.”
When Chen assesses progress in gene therapies for deafness, one of the yardsticks he uses is the progress that has been made in gene therapies for vision loss. Both the eye and the ear occupy small compartments, require relatively low vector doses, and have less intense immune surveillance than other areas of the body. But the treatments for hereditary blindness leapfrogged treatments for deafness six years ago, as demonstrated by the success of Luxturna. The FDA’s approval of the drug was not only a huge boon for gene therapy, but it also created a roadmap for future approvals. But Chen added that trials have failed or been discontinued in the years since Luxturna’s approval. “We are excited,” he remarked, “but we are also very cautious.”
There are more than 150 genes that are known to cause deafness when mutated. (The number of genes involved is thought to be closer to 200–300.) However, many of those are rare; there are about six or seven genes that are the most common causes. According to Chen, the field is taking a “one gene at a time” approach.
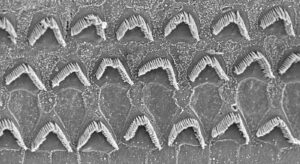
At the moment, many groups are targeting one gene: OTOF. But OTOF mutations are not the most common in hereditary deafness, accounting for 1–8% of genetic hearing loss. (The most common type is DFNB1, caused by mutations in the GJB2 gene, which encodes connexin 26.) So, why OTOF? The target has two big advantages. The first concerns the cells that express OTOF. There are two types of hair cells in the inner ear: the inner hair cells and the outer hair cells. Many AAV vectors can target the inner hair cells, but not the outer hair cells—especially in primates. If a disease affects both types of hair cells, and the delivery vector only targets one type, it won’t work. However, OTOF is expressed only in the inner hair cells, making the gene an attractive target.
The other challenge is that hair cells (and other tissues) degenerate if they’re not functioning properly. And because the ear develops in utero, it is fully mature by the time the baby is born. So, by the time a baby with genetic deafness is born, the cells may have started degenerating, and the window for intervention may have been missed.
But with OTOF mutations, the hair cells don’t degenerate. So, treating years later could restore function. Because of these factors, although OTOF mutations are not the most common mutations, they may be the most treatable. And, in a nascent gene therapy field, a success story is important.
Chen is already thinking about developing treatments for people whose cells have already degenerated. One approach that is currently a major focus of his laboratory is the regeneration of cells that can receive gene therapy. This approach could fundamentally change the equation, but Chen admitted that making the approach work is “a really tall order.”
Basic research for the win
Like Chen, David Corey, PhD, professor at Harvard Medical School, has been interested in the mechanics of hearing for decades—ever since he was a graduate student in the laboratory of Jim Hudspeth, MD, PhD. (Hudspeth is currently a professor at Rockefeller University.) For 50 years, Corey’s research has explored how the inner ear works and how sound is converted into an electrical signal. His laboratory uses basic cell biology techniques including electron microscopy, protein chemistry, and structural biology to assess the proteins and molecular mechanisms underlying hearing.
Recently, Corey made a move into gene therapy that was, perhaps, inevitable. He told GEN that being married to a leader in the gene therapy space means that translational research is a common topic of his dinner conversation. (His wife is Xandra Breakefield, PhD, geneticist, Neurology and Radiology Services, Massachusetts General Hospital.) He laughed recounting the day about seven or eight years ago when Breakefield said to him, “You know so much about these proteins.” And then asked, “When are you going to do something that helps people?”
The decision to make a dedicated move into gene therapy was solidified for Corey on a day in 2017 when he attended a small research symposium on a rare disease—Usher syndrome type 1F—taking place on his campus. Corey said that he wasn’t an invited speaker that day, but he thought it would be a good chance to learn more about a new area. Usher syndrome type 1F, which is characterized by severe hearing loss and progressive blindness, is caused by mutations in protocadherin-15—a protein that Corey was already an expert in. Corey admitted that up until that meeting, he hadn’t thought much about trying to use his knowledge to develop a therapy. But talking to patients at the symposium made him realize that “we had a chance to do something important here.”
Now, seven years later, about two-thirds of Corey’s team works in gene therapy with a large focus on developing a treatment for Usher syndrome type 1F—work led by Maryna Ivanchenko, MD, PhD, an instructor in the Corey lab. The other one-third of the lab continues to work on basic research questions.
“It’s very gratifying,” he said, “that years and years of work on basic science can actually lead to something that could potentially help people.” He pointed out that establishing a crystal structure for protocadherin-15 did more than just add to basic science. It also allowed his lab to engineer a miniature protocadherin-15 that he asserted is currently the best of the different therapeutic strategies. “If we hadn’t had that crystal structure, we wouldn’t have been able to design a miniature version,” he elaborated. “So, very basic science led to a therapeutic strategy.”
Crossing the valley of death
Both Corey and Chen expressed the same sentiment: One of the biggest challenges is how to translate this work to benefit people. Corey refers to the “valley of death” between having a good idea in the lab and a product in the clinic. Part of what makes the valley so forbidding is the sheer cost of running a clinical trial.
But now is the time, they asserted, given the promise and excitement of the recently published results. “We know how things work,” Chen declared. “And we have exciting results from animal to human.”
Some companies have already jumped on board. Decibel Therapeutics, which was acquired by Regeneron in September 2023, reported positive results in October 2023 from the first patient (a child less than 2 years old) dosed in the Phase I/II CHORD trial investigating otoferlin gene therapy (DB-OTO). And in January of this year, the French company Sensorion launched a Phase I/II trial in Europe for its gene therapy SENS-501 for gene-mediated hearing impairment—also targeting otoferlin.


Check out our AAV CDMO service to expedite your gene therapy research
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
[2024/12/20] Gene and Cell Therapy- weekly digest from PackGene
FeaturedNewsArticlesPackGene's NewsletterReceive the latest news and insights to your inbox.About PackGenePackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span...
Sangamo and Astellas Collaborate to Advance Neurological Gene Therapies Using AAV Capsid Technology
Sangamo Therapeutics, Inc. (Nasdaq: SGMO), a leader in genomic medicine, and Astellas Pharma Inc. (TSE: 4503), a global innovator in life sciences, have partnered under a new license agreement. This collaboration centers around Sangamo’s cutting-edge neurotropic AAV...
Inceptor Bio and GRIT Bio Announce Strategic Partnership to Advance IB-T101, a Next-Generation Solid Tumor CAR-T Utilizing the OUTLAST™ Platform
SHANGHAI and MORRISVILLE, N.C., Dec. 18, 2024 /PRNewswire/ -- Inceptor Bio, a leading innovator in cell therapy, and GRIT Bio, a clinical-stage immunotherapy developer, today announced a strategic partnership to advance IB-T101, a potentially best-in-class CAR-T...
Proof-of-concept study bioengineers therapeutics for improved cancer treatment
Credit: Pixabay/CC0 Public DomainA team of Children's Medical Research Institute (CMRI) scientists has identified a new method for producing a therapeutic product that has the potential to improve the treatment of cancer. The work by Associate Professor Leszek...
Related Services

AAV Packaging Services
READ MORE

Off-the-Shelf AAV Products
READ MORE

