The power of CRISPR became definitively clear when the first CRISPR-based gene therapy, Casgevy (exa-cel), won regulatory approvals for the treatment of sickle cell disease. But CRISPR’s applications are not limited to therapeutics. CRISPR has also been used to create preclinical disease models. Indeed, CRISPR provides targeted approaches to systematically process disease-driving genetic mechanisms in model organisms. CRISPR can introduce single-nucleotide variants or knockout genes one at a time, allowing researchers to determine how disease phenotypes or outcomes might depend on individual mutations or even specific combinations of mutations.
CRISPR preclinical models are becoming increasingly attractive because of the vast opportunities to study multiple genetic drivers of disease. Unlike laborious, time-consuming, and costly preclinical genetic knockout methods that involve breeding strategies and the creation of a specific phenotype that allows the study of just one genetic mutation, CRISPR enables the creation of genetically diverse models.
Here, we explore the opportunities and challenges of CRISPR gene editing. It can be used to generate better, more accurate disease models, but it can also produce heterogeneous editing outcomes. Fortunately, this particular challenge can be circumvented with single-cell multiomics technology, as this article will show by using examples from leukemia research.
Single-cell approach to validate genome-edited disease models
CRISPR offers the opportunity to model genetic variances in cancer, allowing scientists to determine how CRISPR-edited genes influence disease progression, drug response, and therapeutic resistance. However, a major hurdle in creating CRISPR preclinical models is that genome editing is heterogeneous. This can lead to variations in zygosity of edits and co-occurring multiplex edits, or unintended outcomes such as off-target edits and chromosomal translocations, necessitating tools that can measure genome edits induced by CRISPR. Such variations are not ideal because they prevent researchers from linking specific edits to drivers of disease and associating a particular genotype to phenotype.
Today, many scientists continue to use traditional bulk analysis of cells to evaluate these CRISPR alterations. The problem is that this conventional analysis is merely an average of the edits within a population of cells. Also, this type of approach does not offer insights into translocations, zygosity, or co-occurrence of mutations at the cell level.
By enriching DNA and protein analyses, single-cell multiomics can provide a powerful approach for validating disease modeling in the earlier stages of discovery. It offers a more comprehensive understanding of the processes underlying diseases.
Case studies of CRISPR-generated cancer models and single-cell approaches
Single-cell multiomics is incredibly important when attempting multiple edits through CRISPR to accurately model the complexity of cancer. The technique enables the analysis of genotype (zygosity status of edits, on-target and predicted off-target edits, mutational co-occurrence of targets in the same cells, and predicted translocation sites), and it provides a clearer understanding of the mutational profile of each edit while also enabling direct phenotype correlation.
In a recent study (ten Hacken et al. Genome Biol. 2020; 21(1): 266), scientists at the Dana-Farber Cancer Institute analyzed single and multiplexed CRISPR edits at the single-cell level to model loss-of-function mutations commonly found in chronic lymphocytic leukemia. The researchers used CRISPR to model loss-of-function mutations in murine hematopoietic cell lines, targeting six commonly mutated genes, namely, Trp53, Atm, Chd2, Samhd1, Mga, and Birc3. Then, using single-cell DNA sequencing, they analyzed single and multiplexed edits in each cell, discovering mutational combinations arising from the edits across the six genes.
The multiplex-edited cells were xenografted into immunocompromised mice, then cells were isolated post-transplant. Interestingly, while pre-transplanted profiles demonstrated a large combinational assortment of edits, post-transplanted cells in vivo showed selection only for Mga and Chd2 mutations, uncovering a specific competitive advantage for these alterations.
The data show how using single-cell approaches can be used to map the location and fitness combination of CRISPR edits. These approaches facilitate the validation of the mutational co-occurrences for each cell, providing a glimpse into the biological complexity of chronic lymphocytic leukemia and an opportunity to develop better-targeted therapeutics for the genetic alterations that drive disease progression.
In another recent study (Iacobucci et al. Blood 2021; 137(12): 1628–1640), St. Jude Children’s Research Hospital scientists generated a novel CRISPR-induced mouse model of acute erythroid leukemia. The model was developed using multiplexed CRISPR editing of mouse hematopoietic stem and progenitor cells, focusing on the combination of mutations found in Trp53, Bcor, Dnmt3a, Rb1, and Nfix, followed by transplant assays.
Using single-cell DNA sequencing, the researchers identified the clonal architecture that led to the propagation and evolution of subsequent tumors, profiling mouse DNA for single-nucleotide variants and indels and identifying co-occurrence at the single-cell level. More specifically, the investigators were able to identify both the primary CRISPR-induced mutations and spontaneous, secondary mutations within the tumors that were driving pathogenesis when cells were serially transplanted into mice.
These two papers are prime examples of how single-cell genotyping can help pinpoint mutational co-occurrence patterns in preclinical models of cancer. Combined with protein analysis, this approach can potentially lead to the creation of better-targeted medicines and more accurate biomarkers.
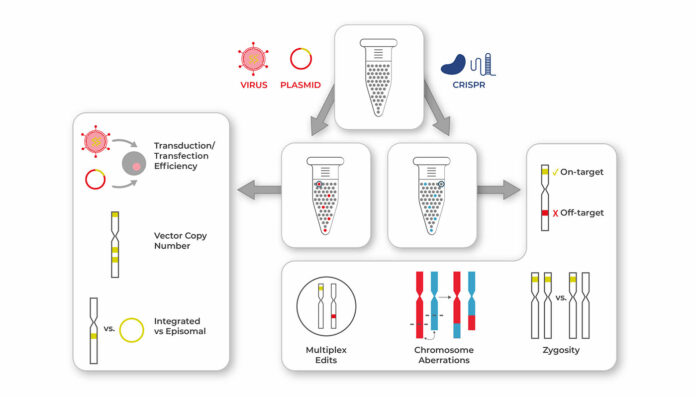
Inconsistencies that occur in cells that are being prepared for use in cell therapies can be assessed with Mission Bio’s Tapestri platform. For cell therapies involving the introduction of a transgene, Tapestri enables gene transfer measurements such as transduction/transfection efficiency. For therapies modified by CRISPR or other gene editing tools, Tapestri can concomitantly assess sources of variability such as on- and off-target editing.
Future of single-cell CRISPR approaches
Beyond validating CRISPR preclinical cancer models for early-stage discovery, single-cell approaches can be used for validation across the entire drug development continuum. For example, after confirming edits in disease models, single-cell analysis can be leveraged for later-stage translational genome editing studies to help optimize drug targets.
In clinical phases, when comprehensive analysis of genome-edited therapies is necessary (such as when it is required by regulatory agencies), single-cell multiomics can be utilized for measuring potential early indicators of potency, safety, and efficacy.For gene transfer, single-cell multiomics offers the capability to evaluate the extent of cell transduction and to determine vector copy number.
CRISPR approaches are also making waves in the development of preclinical models of rare diseases, such as cystic fibrosis and Niemann-Pick disease type C1 (Pradhan et al. Curr. Stem Cell Rep. 2020; 6(3): 41–51). Tapping into single-cell validation will play a role in revealing the complex genetic nature of such diseases and supporting drug development.
Single-cell multiomics is becoming a powerful tool for analyzing DNA and protein, revealing the intricate details at the cellular level at a resolution that other methods cannot capture. Because it elucidates and deeply characterizes the heterogeneity of genome edited disease models and the editing efficacy of cell and gene therapies, single-cell analyses can dramatically advance translational research

Check out our AAV CDMO service to expedite your gene therapy research
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
[2024/12/20] Gene and Cell Therapy- weekly digest from PackGene
FeaturedNewsArticlesPackGene's NewsletterReceive the latest news and insights to your inbox.About PackGenePackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span...
Sangamo and Astellas Collaborate to Advance Neurological Gene Therapies Using AAV Capsid Technology
Sangamo Therapeutics, Inc. (Nasdaq: SGMO), a leader in genomic medicine, and Astellas Pharma Inc. (TSE: 4503), a global innovator in life sciences, have partnered under a new license agreement. This collaboration centers around Sangamo’s cutting-edge neurotropic AAV...
Inceptor Bio and GRIT Bio Announce Strategic Partnership to Advance IB-T101, a Next-Generation Solid Tumor CAR-T Utilizing the OUTLAST™ Platform
SHANGHAI and MORRISVILLE, N.C., Dec. 18, 2024 /PRNewswire/ -- Inceptor Bio, a leading innovator in cell therapy, and GRIT Bio, a clinical-stage immunotherapy developer, today announced a strategic partnership to advance IB-T101, a potentially best-in-class CAR-T...
Proof-of-concept study bioengineers therapeutics for improved cancer treatment
Credit: Pixabay/CC0 Public DomainA team of Children's Medical Research Institute (CMRI) scientists has identified a new method for producing a therapeutic product that has the potential to improve the treatment of cancer. The work by Associate Professor Leszek...
Related Services

AAV Packaging Services
READ MORE

Off-the-Shelf AAV Products
READ MORE

