In a pioneering study published in *Science*, a team of researchers led by Peter H. Yoon and Jennifer A. Doudna from the University of California, Berkeley, has made a remarkable discovery in the realm of CRISPR technology. The team has identified an ancestral clade of CRISPR-Cas13 ribonucleases, shedding new light on the evolutionary origins of these RNA-guided enzymes.
The Ancestral CRISPR-Cas13 Discovery
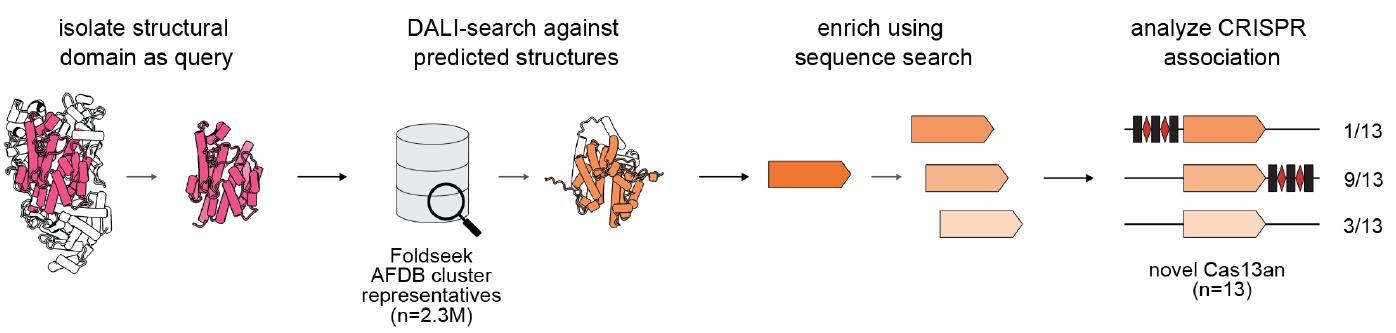
CRISPR-Cas13 systems, known for their role in bacterial adaptive immunity and programmable RNA manipulation, have always posed a challenge due to their limited sequence similarity. To overcome this, the Berkeley team developed an automated structural-search pipeline. This innovative approach led to the identification of an ancestral form of Cas13, termed Cas13an, which exhibits unique structural and functional properties.
Cas13an is significantly smaller than its known counterparts, comprising just one-third of the size of other Cas13 enzymes. Despite its compact structure, Cas13an maintains robust programmable RNA depletion capabilities and provides defense against a diverse array of bacteriophages. Unlike other Cas13 systems, Cas13an uses a single active site for both CRISPR RNA processing and RNA-guided cleavage, highlighting its dual functionality.
Evolutionary Insights and Functional Implications
The discovery of Cas13an offers profound insights into the evolution of CRISPR-Cas systems. The research indicates that Cas13an likely represents an early evolutionary form of Cas13, tracing its origins back to defense-associated ribonucleases. The team’s phylogenetic analyses suggest that all three major Class 2 CRISPR-Cas effectors—Cas9, Cas12, and Cas13—originated from compact ancestral proteins that gradually evolved through domain accretion.
Cas13an’s structure is characterized by the absence of large insertions in its HEPN domains and the lack of a canonical REC lobe, features that are reminiscent of smaller Cas9 and Cas12 proteins and their ancestral forms. This compact architecture underlines the evolutionary trajectory of CRISPR-Cas systems, providing a clearer understanding of how these enzymes have diversified over time.
Potential for Precision RNA Editing
The functional versatility of Cas13an opens new avenues for precision RNA editing. In experimental setups using *E. coli* as a heterologous host, Cas13an demonstrated effective RNA interference and protection against phage infection. Its ability to cleave both RNA targets and process CRISPR arrays into functional crRNAs underscores its potential as a versatile tool for RNA manipulation.
The study’s findings also reveal that Cas13an’s HEPN domains play a crucial role in both target RNA cleavage and pre-crRNA processing. This multifunctionality suggests that ancestral CRISPR-Cas enzymes may have utilized a single active site for multiple ribonucleolytic activities, a trait that could be harnessed for developing more efficient and compact CRISPR tools.
Future Directions and Applications
The Berkeley team’s discovery paves the way for future exploration of the evolution and mechanisms of RNA-guided ribonuclease activity. By leveraging structure-guided protein mining, researchers can uncover homologies between highly divergent proteins, enhancing our understanding of biomolecular evolution.
As structural prediction methods and databases continue to advance, the potential for discovering new CRISPR-Cas systems and other biologically significant enzymes will expand, offering new tools for biotechnology and therapeutic applications.
The identification of Cas13an marks a significant milestone in CRISPR research, bridging gaps in our understanding of CRISPR-Cas system origins and expanding the toolkit available for precision genome and transcriptome engineering. This discovery not only deepens our comprehension of molecular evolution but also heralds new possibilities for the development of innovative RNA-based technologies.
https://www.science.org/doi/abs/10.1126/science.adq0553

Check out our AAV CDMO service to expedite your gene therapy research
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
Genethon Highlights Key Developments in Gene Therapy Research
December 19, 2024 – Paris, France – Genethon, a pioneering non-profit gene therapy research and development organization founded by the French Muscular Dystrophy Association (AFM-Telethon), has unveiled significant progress in its clinical and scientific endeavors as...
[2024/12/20] Gene and Cell Therapy- weekly digest from PackGene
FeaturedNewsArticlesPackGene's NewsletterReceive the latest news and insights to your inbox.About PackGenePackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span...
Sangamo and Astellas Collaborate to Advance Neurological Gene Therapies Using AAV Capsid Technology
Sangamo Therapeutics, Inc. (Nasdaq: SGMO), a leader in genomic medicine, and Astellas Pharma Inc. (TSE: 4503), a global innovator in life sciences, have partnered under a new license agreement. This collaboration centers around Sangamo’s cutting-edge neurotropic AAV...
Inceptor Bio and GRIT Bio Announce Strategic Partnership to Advance IB-T101, a Next-Generation Solid Tumor CAR-T Utilizing the OUTLAST™ Platform
SHANGHAI and MORRISVILLE, N.C., Dec. 18, 2024 /PRNewswire/ -- Inceptor Bio, a leading innovator in cell therapy, and GRIT Bio, a clinical-stage immunotherapy developer, today announced a strategic partnership to advance IB-T101, a potentially best-in-class CAR-T...
Related Services

Plasmids GMP Services

AAV GMP Services


