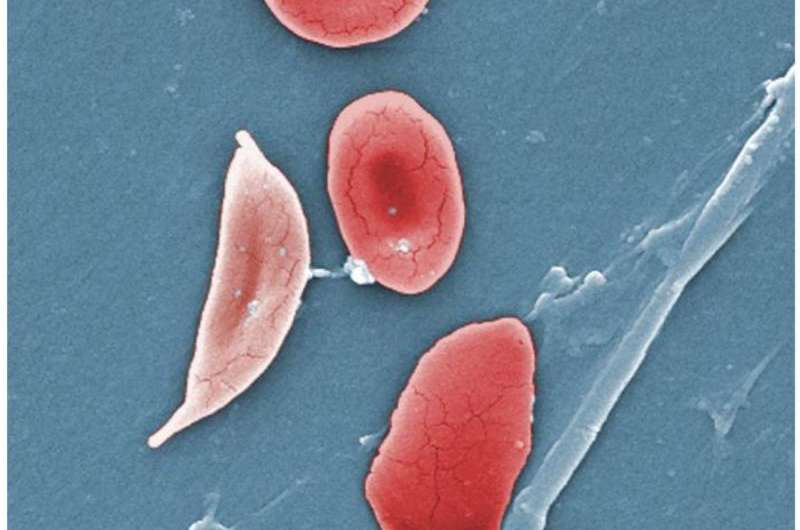
Normal blood cells next to a sickle-blood cell, colored scanning electron microscope image. Credit: Wikipedia/Illustration from Anatomy & Physiology
New research published in the New England Journal of Medicine indicates that stem cell gene therapy may offer a promising, curative treatment for the painful, inherited blood disorder sickle cell disease (SCD).
The findings from a new clinical trial, published August 31, add to the body of evidence supporting gene therapy as a treatment for sickle cell disease, which primarily impacts people of color.
About 100,000 Americans have sickle cell disease, according to the U.S. Centers for Disease Control and Prevention. The condition, which can cause a lifetime of pain, health complications and expenses, affects one in 365 Black babies born in the U.S. and one in 16,300 Hispanic babies.
Until recently, the only treatment options have been intensive bone marrow transplants from siblings or matched donors. But other curative therapies are now on the horizon. The University of Chicago Medicine Comer Children’s Hospital was one of three sites to enroll patients in the clinical trial, which tested a stem cell gene therapy to treat sickle cell disease.
As part of the trial, researchers used CRISPR-Cas9 to edit specific genes in stem cells—the building blocks of blood cells—taken from each patient. The edits increased the cells’ production of fetal hemoglobin (HbF), a protein that can replace unhealthy, sickled hemoglobin in the blood and protect against the complications of sickle cell disease. The patients then received their own edited cells as therapeutic infusions.
The therapy was the second for this disease to use CRISPR-Cas9 technology and the first to target a new genetic area and use cryopreserved stem cells with the hope of increasing access to such a treatment. Other gene therapy studies for SCD have used lentiviruses—a type of virus often modified and used for gene editing which remain in the cell long-term. No foreign material remains in stem cells edited with CRISPR-Cas9.
Trial participants who received the CRISPR-edited stem cells reported a decrease in vaso-occlusive events, a painful phenomenon that occurs when sickled red blood cells accumulate and cause a blockage.
“The biggest take-home message is that there are now more potentially curative therapies for sickle cell disease than ever before that lie outside of using someone else’s stem cells, which can bring a host of other complications,” said James LaBelle, MD, Ph.D., director of the Pediatric Stem Cell and Cellular Therapy Program at UChicago Medicine and Comer Children’s Hospital and senior author of the study.
“Especially in the last 10 years, we’ve learned about what to do and what not to do when treating these patients. There’s been a great deal of effort towards offering patients different types of transplants with decreased toxicities, and now gene therapy rounds out the set of available treatments, so every patient with sickle cell disease can get some sort of curative therapy if needed. At UChicago Medicine, we’ve built infrastructure to support new approaches to sickle cell disease treatment and to bring additional gene therapies for other diseases.”
As the scientific community continues to refine and expand the applications of gene therapy, the potential for curative treatments for diseases like sickle cell disease is becoming more of a transformative reality. The journey is ongoing, with the need for long-term follow-up and further research, but this study provides an encouraging glimpse into a future of effective genetic interventions.
In the larger context of therapeutic development, LaBelle stressed the importance of the study’s contribution to the growing body of evidence supporting the viability of gene therapy as a treatment for sickle cell disease. Two other gene therapies for the disease are awaiting FDA approval this year.
“The data from this trial supports bringing on similar gene therapies for sickle cell disease and for other bone marrow-derived diseases. If we didn’t have this data, those wouldn’t move forward,” he said.
Check out our AAV capsid engineering service to expedite your gene therapy research
PackGene is a CRO & CDMO technology company that specializes in packaging recombinant adeno-associated virus (rAAV) vectors. Since its establishment in 2014, PackGene has been a leader in the AAV vector CRO service field, providing tens of thousands of custom batches of AAV samples to customers in over 20 countries. PackGene offers a one-stop CMC solution for the early development, pre-clinical development, clinical trials, and drug approval of rAAV vector drugs for cell and gene therapy (CGT) companies that is fast, cost-effective, high-quality, and scalable. Additionally, the company provides compliant services for the GMP-scale production of AAVs and plasmids for pharmaceutical companies, utilizing five technology platforms, including the π-Alpha™ 293 cell AAV high-yield platform and the π-Omega™ plasmid high-yield platform. PackGene’s mission is to make gene therapy affordable and accelerate the launch of innovative gene drugs. The company aims to simplify the challenging aspects of gene therapy development and industrialization processes and provide stable, efficient, and economical rAAV Fast Services to accelerate gene and cell therapy development efforts from discovery phase to commercialization.
Related News
Genethon Highlights Key Developments in Gene Therapy Research
December 19, 2024 – Paris, France – Genethon, a pioneering non-profit gene therapy research and development organization founded by the French Muscular Dystrophy Association (AFM-Telethon), has unveiled significant progress in its clinical and scientific endeavors as...
[2024/12/20] Gene and Cell Therapy- weekly digest from PackGene
FeaturedNewsArticlesPackGene's NewsletterReceive the latest news and insights to your inbox.About PackGenePackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span...
Sangamo and Astellas Collaborate to Advance Neurological Gene Therapies Using AAV Capsid Technology
Sangamo Therapeutics, Inc. (Nasdaq: SGMO), a leader in genomic medicine, and Astellas Pharma Inc. (TSE: 4503), a global innovator in life sciences, have partnered under a new license agreement. This collaboration centers around Sangamo’s cutting-edge neurotropic AAV...
Inceptor Bio and GRIT Bio Announce Strategic Partnership to Advance IB-T101, a Next-Generation Solid Tumor CAR-T Utilizing the OUTLAST™ Platform
SHANGHAI and MORRISVILLE, N.C., Dec. 18, 2024 /PRNewswire/ -- Inceptor Bio, a leading innovator in cell therapy, and GRIT Bio, a clinical-stage immunotherapy developer, today announced a strategic partnership to advance IB-T101, a potentially best-in-class CAR-T...
Related Services

Plasmids GMP Services
Multiple scales & grade of solutions of various kind of plasmids suitable for multiple treatments in a fast and cost effective way.
READ MORE

AAV GMP Services
Ranging from small-scale AAV production, to large-scale AAV cGMP manufacturing for animal studies.
READ MORE

Technology Platforms
PackGene’s proprietary π-Alpha™ 293 AAV High-yield Platform increases AAV production by 3 to 8 times that of traditional platforms.
READ MORE

