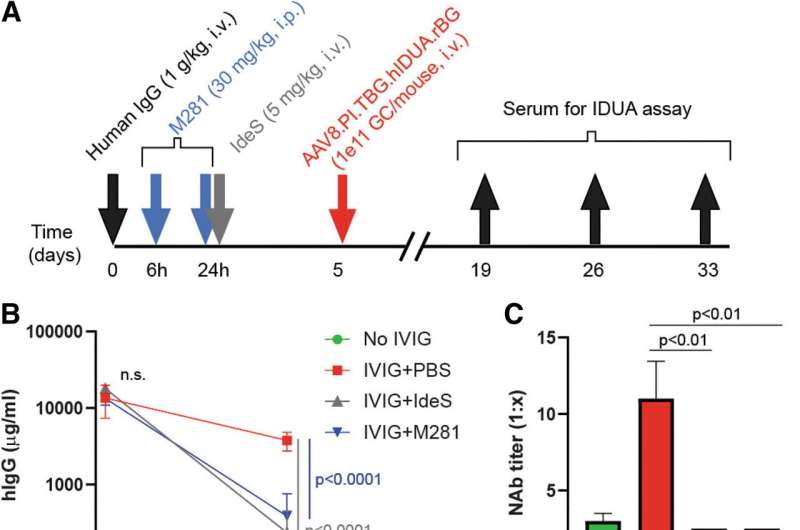
M281 treatment prevents human NAb-mediated reduction of liver-targeted gene delivery after intravenous AAV administration in humanized FcRn transgenic mice. (A) Scheme of the in-mouse study assessing M281 effects on hIgG and anti-AAV NAb levels and subsequent systemic vector transduction to the liver comparing those by IdeS in hFcRnTg32.scid mice pretreated with hIgG. (B) hIgG concentrations in mouse serum samples over time. hIgG was undetectable in the No IVIG group (n = 5 per group). (C) AAV8 NAb titers at day 5 post-hIgG (n = 5 per group). (D) Serum IDUA activity post-AAV8.TBG.hIDUA.rBG vector administration. Data were analyzed using ANOVA followed by a post hoc Tukey test. AAV, adeno-associated virus; ANOVA, analysis of variance; FcRn, neonatal Fc receptor; GC, genome copy; hIgG, human IgG; IdeS, imlifidase; IDUA, α-I-iduronidase; i.p., intraperitoneal; i.v., intravenous; IVIG, intravenous immunoglobulin; NAb, neutralizing antibody; PBS, phosphate-buffered saline; TBG, thyroid hormone-binding globulin promoter. Credit: Human Gene Therapy (2023). DOI: 10.1089/hum.2022.216
AAV vectors represent the leading platform for gene delivery to treat rare genetic disorders. However, the prevalence of natural humoral immunity, with Nabs, prevents a significant proportion of the population from being treated with AAV-based gene therapy. IgG appears to be the predominant antibody class among Nabs. Inhibitors of the neonatal Fc receptor (FcRn) represent one group of IgG-reducing drugs in clinical development. M281 is a anti-human FcRn monoclonal antibody that can reduce IgG levels to less than 20% of the baseline level.
In the current study, James M. Wilson, MD, Ph.D., from the Perelman School of Medicine, University of Pennsylvania, and co-authors, evaluated the effect of M281-mediated IgG reduction on pre-existing NAb titers and gene transduction in the context of systemic AAV-based gene therapy in mice and non-human primates.
Based on their results, the investigators concluded that “mitigating pre-existing humoral immunity via disruption of the neonatal Fc receptor–IgG interaction may make adeno-associated virus-based gene therapies effective in neutralizing antibody-positive patients.”
“The presence of neutralizing antibodies to AAV can disqualify some patients from receiving gene therapy,” says Editor-in-Chief Terence R. Flotte, MD, Celia and Isaac Haidak Professor of Medical Education and Dean, Provost, and Executive Deputy Chancellor, University of Massachusetts Chan Medical School. “This intervention has the potential to circumvent that limitation and allow more patients to benefit from gene therapy in the future.”
PackGene is a world-leading AAV vector CRO and CDMO company. We provides economical, reliable, and scalable plasmid DNA and AAV viral vector production for early-stage drug discovery, preclinical development and clinical trials for Cell and Gene Therapy (CGT). If you want more information about our AAV services, please contact us.

