A new research paper titled “AAV1.NT-3 gene therapy prevents age-related sarcopenia” has been published in Aging.
Sarcopenia is progressive loss of muscle mass and strength occurring during normal aging with significant consequences on the quality of life for elderly. Neurotrophin 3 (NT-3) is an important autocrine factor supporting Schwann cell survival and differentiation and stimulating axon regeneration and myelination. NT-3 is involved in the maintenance of neuromuscular junction (NMJ) integrity, restoration of impaired radial growth of muscle fibers through activation of the Akt/mTOR pathway.
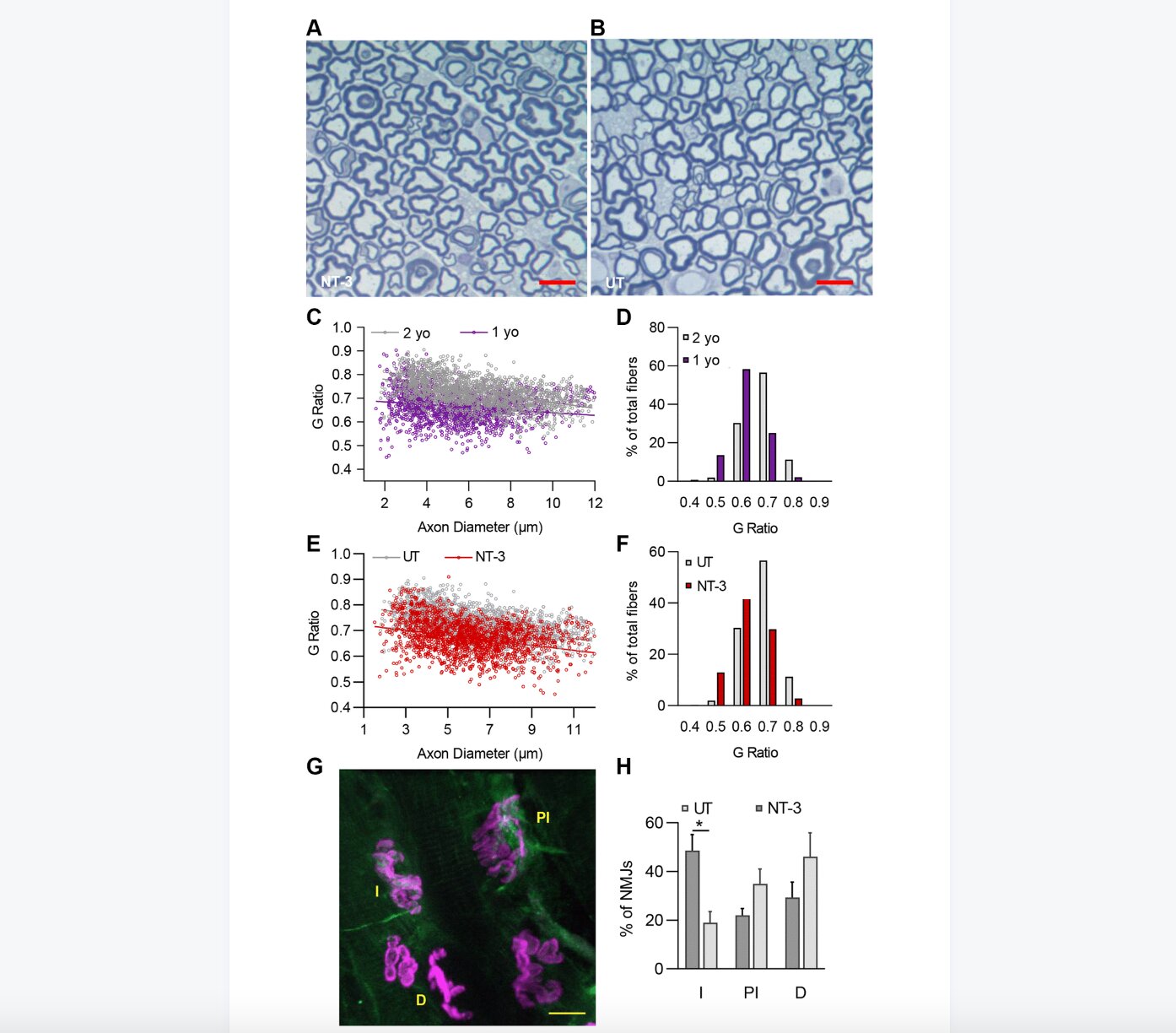
In this new study, researchers Burcak Ozes, Lingying Tong, Morgan Myers, Kyle Moss, Alicia Ridgley, and Zarife Sahenk from Nationwide Children’s Hospital and The Ohio State University used a triple muscle-specific creatine kinase (tMCK) promoter to restrict NT-3 expression to the skeletal muscle and self-complimentary adeno-associated virus serotype 1 (scAAV1) as vector to assess the therapeutic efficacy of AAV1.NT-3 in wild type-aged C57BL/6J mice, a model for natural aging and sarcopenia.
“Quantitative histopathologic parameters served to address age-related changes in muscle, peripheral nerve and NMJ,” the researchers write.
The treatment efficacy was assessed at 6 months post-injection using run-to-exhaustion and rotarod tests, in vivo muscle contractility assay, and histopathological studies of the peripheral nervous system, including NMJ connectivity and muscle. AAV1.NT-3 gene therapy in WT-aged C57BL/6 mice resulted in functional and in vivo muscle physiology improvements, supported by quantitative histology from muscle, peripheral nerves and NMJ.
Hindlimb and forelimb muscles in the untreated cohort showed the presence of a muscle- and sex-dependent remodeling and fiber size decrease with aging, which was normalized toward values obtained from 10 months old WT mice with treatment. The molecular studies assessing the NT-3 effect on the oxidative state of distal hindlimb muscles, accompanied by western blot analyses for mTORC1 activation were in accordance with the histological findings.
“When considering the burden of sarcopenia on the lifestyle of elderly, and on the healthcare system, we believe this preclinical study is providing strong support for AAV.NT-3 gene therapy in the successful management of sarcopenia, as a serious and plausible option in the future,” the researchers note.
From Medical Express: https://medicalxpress.com/news/2023-03-team-aav1nt-gene-therapy-age-related.html
PackGene is a world-leading AAV vector CRO and CDMO company. We provides economical, reliable, and scalable plasmid DNA and AAV viral vector production for early-stage drug discovery, preclinical development and clinical trials for Cell and Gene Therapy (CGT). If you want more information about our AAV services, please contact us.

