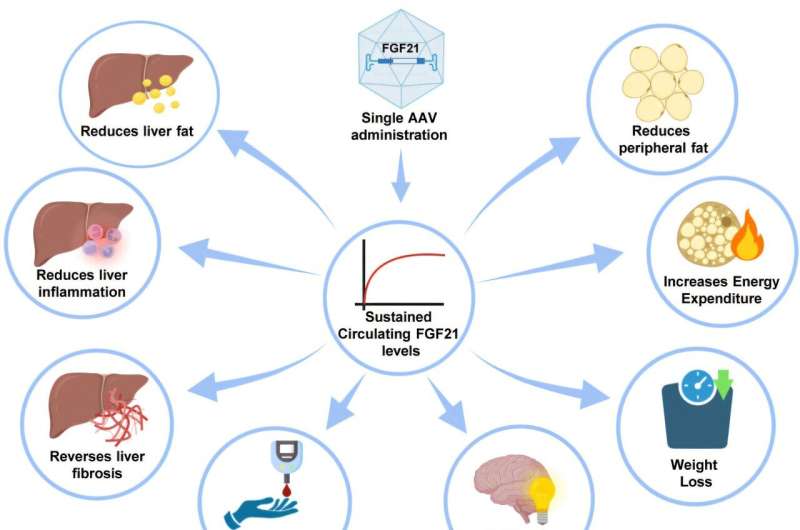
UAB researchers have reversed metabolic dysfunction-associated steatohepatitis (MASH) in mouse models. MASH is a severe liver disease associated with obesity and type 2 diabetes that affects more than 40 million people. The results were obtained with a single intramuscular administration of the therapeutic viral vectors.
The research has also determined that most obese, type 2 diabetic and MASH patients could benefit from the therapy. The results, published in Molecular Therapy, will be the basis for a future clinical trial by the biopharmaceutical company Kriya.
Researchers from the Universitat Autònoma de Barcelona (UAB) in collaboration with clinicians from the Parc Taulí University Hospital in Sabadell have described in mice the long-term efficacy and safety of the intramuscular administration of a gene therapy for the treatment of MASH, a liver disease that affects approximately 40 million people in the United States and Europe.
The therapy developed by the UAB researchers is based on the genetic engineering of the skeletal muscle with the gene that encodes for the fibroblast growth factor 21 protein using adeno-associated viral vectors (AAV-FGF21 vectors). FGF21 is a key metabolic regulator that, upon the administration of the vectors in skeletal muscle, is sustainedly increased in bloodstream (more than a year in this study).
This treatment mediates long-term reversal of liver fibrosis and MASH, counteracts obesity, excessive fat accumulation, insulin resistance characteristic of type 2 diabetes, and the development of liver tumors both in female and male mice.
To facilitate clinical translation, the researchers have evaluated this therapy in dogs to assess the safety and therapeutic benefit in large animals. They have also characterized FGF21 circulating levels in a cohort of 500 obese, type 2 diabetes, and MASH patients, and conclude that most of them would be eligible for this gene therapy in the future.
The results will be the basis for a future clinical trial. The UAB licensed this gene therapy program with AAV-FGF21 to the company Tramontane Therapeutics Inc., now part of the biopharmaceutical company Kriya, which will bring this approach to the clinic in human MASH patients.
“Our gene therapy based on AAV-FGF21 can be transformative for patients with MASH, a disease that requires safe, effective and long-lasting treatments,” explains UAB researcher Fatima Bosch, who led the research.
Obesity and type 2 diabetes, precursors of MASH
The global epidemics of obesity and type 2 diabetes are risk factors for the development of liver diseases. Metabolic dysfunction-associated steatotic liver disease (MASLD), or “fatty liver disease,” is the most common chronic liver disease worldwide, an epidemic whose prevalence can reach 27% of the adult population in some countries.
It begins with an excessive accumulation of lipids in the liver that can worsen into severe metabolic dysfunction-associated steatohepatitis (MASH), characterized by inflammation, lesions in the liver cells (hepatocytes) and fibrosis. In advanced stages, MASH is associated with severe liver diseases, such as cirrhosis, liver cancer and end-stage liver disease, with high mortality.
“The gene therapy strategy we have developed could be a major advance in the treatment not only of patients with MASH, but also of other metabolic diseases and related comorbidities that affect millions of people worldwide,” says Dr. Bosch.

Check out our AAV CDMO service to expedite your gene therapy research
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
[2024/12/20] Gene and Cell Therapy- weekly digest from PackGene
FeaturedNewsArticlesPackGene's NewsletterReceive the latest news and insights to your inbox.About PackGenePackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span...
Sangamo and Astellas Collaborate to Advance Neurological Gene Therapies Using AAV Capsid Technology
Sangamo Therapeutics, Inc. (Nasdaq: SGMO), a leader in genomic medicine, and Astellas Pharma Inc. (TSE: 4503), a global innovator in life sciences, have partnered under a new license agreement. This collaboration centers around Sangamo’s cutting-edge neurotropic AAV...
Inceptor Bio and GRIT Bio Announce Strategic Partnership to Advance IB-T101, a Next-Generation Solid Tumor CAR-T Utilizing the OUTLAST™ Platform
SHANGHAI and MORRISVILLE, N.C., Dec. 18, 2024 /PRNewswire/ -- Inceptor Bio, a leading innovator in cell therapy, and GRIT Bio, a clinical-stage immunotherapy developer, today announced a strategic partnership to advance IB-T101, a potentially best-in-class CAR-T...
Proof-of-concept study bioengineers therapeutics for improved cancer treatment
Credit: Pixabay/CC0 Public DomainA team of Children's Medical Research Institute (CMRI) scientists has identified a new method for producing a therapeutic product that has the potential to improve the treatment of cancer. The work by Associate Professor Leszek...
Related Services

AAV Packaging Services
READ MORE

Off-the-Shelf AAV Products
READ MORE

