Adeno-associated virus (AAV) is a non-pathogenic single-stranded DNA virus that is an exceptional research tool as well as an attractive candidate for genetic payload delivery in Gene and Cell Therapies.
Several AAV features position them as exceptional research tools as well as attractive candidates for genetic payload delivery in Gene and Cell Therapies. Notable features include: (1) AAV are not currently known to cause any disease, (2) AAV infection results in a very mild immune response, (3) AAV are capable of infecting both dividing and non-dividing cells, and (4) recombinant AAV (rAAV) are capable of driving prolonged expression of a gene of interest (GOI) without integrating the GOI into the host genome. In addition, differences in the capsid structure of various AAV serotypes bias infection rates across host tissue and cell-types and therefore provide a potential mechanism for tissue or cell-type infection specificity.
As a result, our growing base of satisfied customers repeatedly relies on us for their AAV vector cloning and packaging needs. Beyond Research, NHP, and HT-grade AAV for your pre-clinical studies, we also offer GMP-grade AAV manufacturing for clinical applications.
Fast Turnaround
High Quality
Serotype Variety
Proven Expertise
10,000+ custom AAV projects completed
Vector Design Support
AAV grades from research to GMP
Comprehensive Solutions
| PackGene AAV Packaging Service | |||
|---|---|---|---|
| Research Grade | NHP Grade | HT Grade | |
| Application | Cell culture, small animal | Small animal or non-human primates | Cell culture |
| Scale | 2E+12 ~ 8E+14 GC | 1E+13 ~ 1E+16 | 2E+11 ~ 2E+13 GC |
| Lead time | Start from 12 business days | Start from 17 business days | 7-14 business days |
| QC test | qPCR, SDS-PAGE Coomassie Blue Staining, endotoxin LAL, plasmid restriction enzyme digestion | ddPCR, SDS-PAGE Silver Staining, endotoxin LAL, plasmid restriction enzyme digestion | qPCR |
| Performance Comparison | |||
| Titration | qPCR, Titer meet requirements | ddPCR, Titer meet requirements | qPCR, Titer meet requirements |
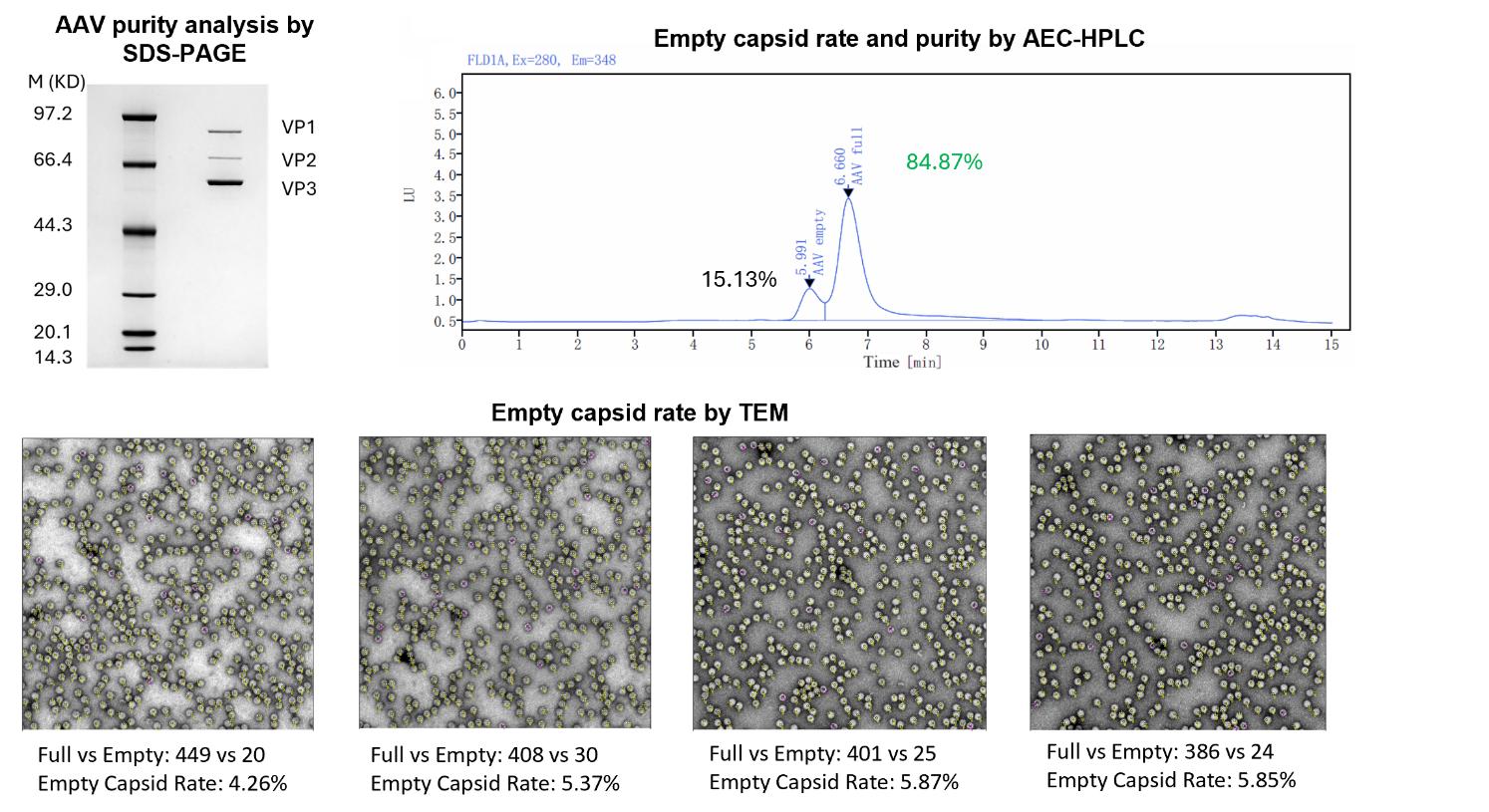
| AAV purity analysis | SDS-PAGE, Coomassie Blue Staining, Capsid band size meet reference | SDS-PAGE, Silver Staining, Capsid band size meet reference, Capsid band size meet reference | - |
| Endotoxin Test | LAL, <10EU/mL | LAL, <1EU/ml (endotoxin removal may reach <0.2EU/ml) | - |
| Mycoplasma | - | qPCR, Negative | - |
| Bioburden | - | 48 hr, no growth | - |
| AAV Genome integrity | - | Capillary Electrophoresis, report value | - |
| Empty Capsid | TEM, guaranteed empty capsid rate <30% for common serotypes | TEM, guaranteed empty capsid rate <20% for common serotypes | - |
| TEM images | 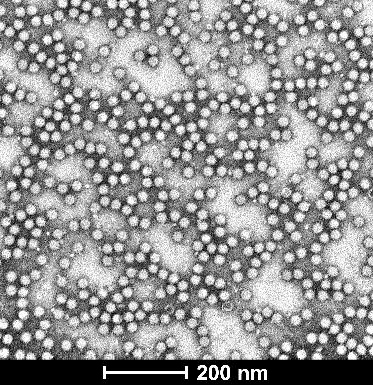 |
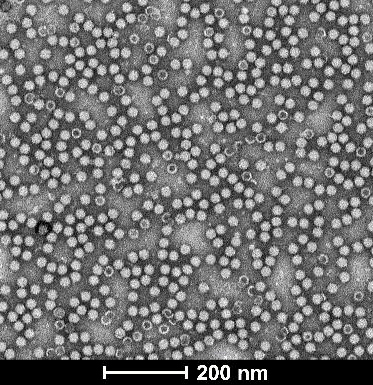 |
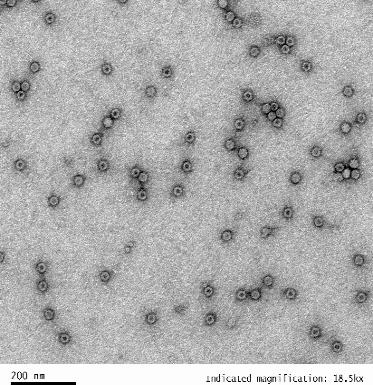 |
We offer more than 40 standarized AAV quality assays.
AAV Packaging – Research Grade
READ MORE
AAV Packaging – NHP Grade
READ MORE
AAV Packaging – HT Grade
READ MORE