*Nicolás Sandoval-Villegas, Zoltán Ivics, The best of both worlds: AAV-mediated gene transfer empowered by LNP delivery of Sleeping Beauty transposase for durable transgene expression in vivo, Molecular Therapy, Volume 32, Issue 10, 2024, Pages 3211-3214, ISSN 1525-0016, https://doi.org/10.1016/j.ymthe.2024.09.002.
(https://www.sciencedirect.com/science/article/pii/S1525001624005896)
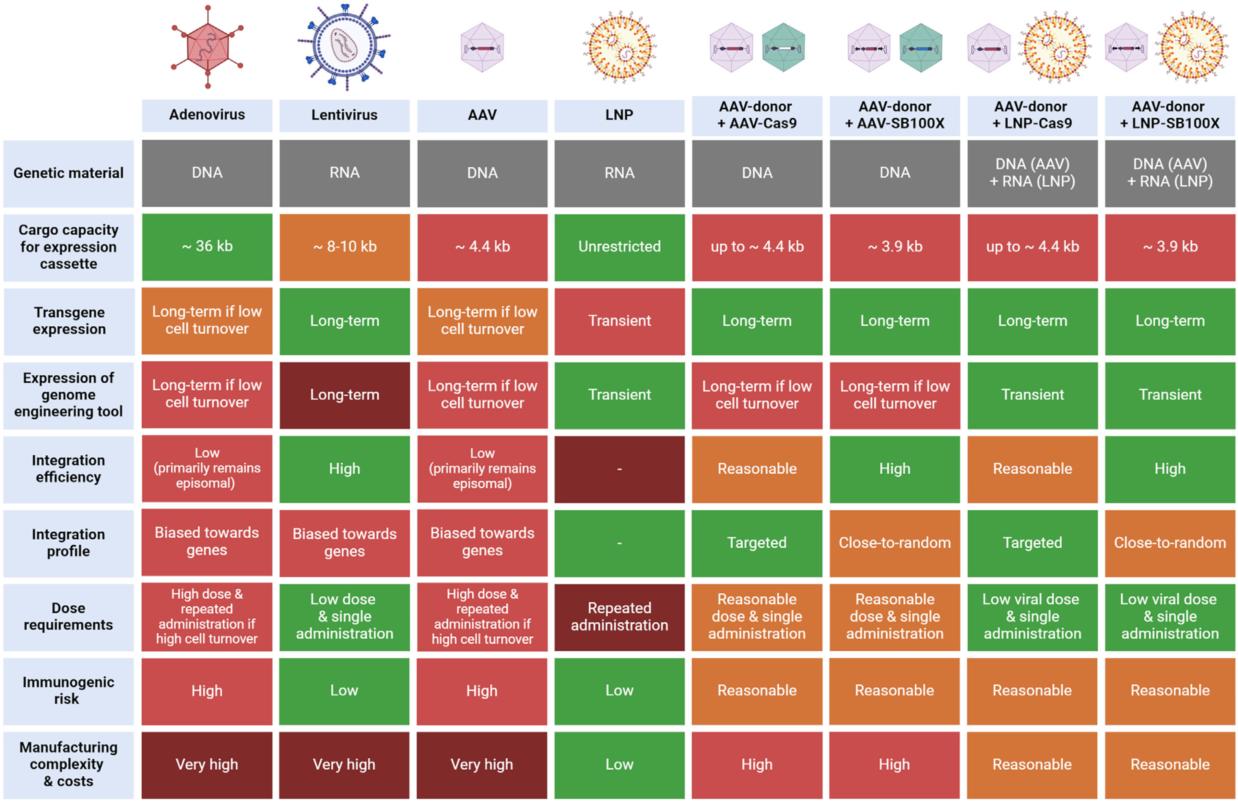
Liver-targeted gene therapies are at the forefront of treating genetic diseases, particularly with the development of adeno-associated virus (AAV) and Sleeping Beauty (SB) transposon systems. These technologies offer a range of benefits for both pediatric and adult patients, including efficient and stable genome integration. The SB transposon system has demonstrated a genome-wide integration profile, making it an attractive tool for gene therapies. When combined with AAV vectors, it enhances the potential for precise and stable gene delivery, particularly for liver-targeted applications.
AAV vectors are widely used in gene therapy due to their relatively low immunogenicity and ability to target specific tissues, such as the liver. However, AAV vectors are limited by their cargo capacity, which can restrict the size of the therapeutic gene they can carry. To overcome this limitation, hybrid vector systems have been developed, combining the advantages of AAV with the genomic integration capabilities of the SB transposon system.
Recent studies have explored alternative delivery methods for the SB transposon system, including the use of polyethylenimine (PEI) to form DNA complexes for intravenous injection, targeting the lungs. Physical delivery methods like hydrodynamic injection have also been employed for liver-targeted gene delivery, ensuring efficient transposon transfer.
The most promising advancement in this field is the development of AAV-SB hybrid vectors. These hybrid systems take advantage of the natural ability of viruses to penetrate cell membranes while enabling stable genomic integration via the SB transposon system. Zakas et al. described a hybrid vector approach incorporating SB transposon components into various viral vectors, including integrase-defective lentiviral particles, adenovirus vectors, herpes simplex virus vectors, and baculovirus vectors. This combination allows for the efficient transfer of large genetic payloads into target cells.
One innovative application of these hybrid vectors is in the targeting of hematopoietic stem cells (HSCs) in vivo. Researchers have demonstrated that autologous HSCs can be mobilized into peripheral blood and genetically engineered using a hybrid adenovirus/SB transposon vector system. This approach has led to functional, genetically modified HSCs in humanized mouse models, showing great potential for future clinical applications.
AAV vectors, although limited in cargo capacity compared to adenoviral vectors, remain a critical tool in gene therapy due to their well-established safety profile and their ability to achieve tissue-specific delivery. Hybrid AAV-SB vectors offer a solution to the cargo limitations of AAV, while still benefiting from the transposon’s ability to mediate efficient genomic integration. In contrast, other methods, such as CRISPR-Cas9, rely on double-strand DNA breaks and repair mechanisms, which carry risks such as off-target effects and chromosomal translocations.
The safety of AAV-SB hybrid systems, particularly in terms of genomic integration, remains a topic of ongoing research. While the SB transposon system is considered to have a random integration profile, this randomness may reduce the risk of insertional mutagenesis compared to retroviral or lentiviral vectors, which often integrate near active genes. However, careful evaluation of the genomic integration sites is required to ensure long-term safety in clinical applications.
The use of AAV-SB transposon hybrid systems for liver-targeted gene therapies represents a promising advancement in the field of genetic medicine. These hybrid systems combine the safety and targeting advantages of AAV with the stable integration capabilities of the SB transposon system, potentially providing new treatment options for genetic liver diseases. Future research will focus on optimizing the delivery, safety, and efficacy of these hybrid vectors to ensure their successful transition from experimental models to clinical therapies.
Another promising approach for gene editing applications is PackGene’s SB-100 mRNA system. SB-100 mRNA offers an efficient and non-viral method for delivering the Sleeping Beauty transposon system into target cells, facilitating precise gene integration without the need for viral vectors. This method enhances safety by eliminating concerns related to viral vector immunogenicity, while maintaining the high efficiency of transposon-mediated integration. PackGene’s SB-100 mRNA is especially valuable for gene editing in liver-targeted therapies, as it can deliver larger genetic payloads, circumventing the cargo limitations associated with AAV vectors. As part of PackGene’s expanding mRNA service portfolio, this system represents a cutting-edge tool for advancing both preclinical and clinical gene editing applications.
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
[2024/12/20] Gene and Cell Therapy- weekly digest from PackGene
FeaturedNewsArticlesPackGene's NewsletterReceive the latest news and insights to your inbox.About PackGenePackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span...
Sangamo and Astellas Collaborate to Advance Neurological Gene Therapies Using AAV Capsid Technology
Sangamo Therapeutics, Inc. (Nasdaq: SGMO), a leader in genomic medicine, and Astellas Pharma Inc. (TSE: 4503), a global innovator in life sciences, have partnered under a new license agreement. This collaboration centers around Sangamo’s cutting-edge neurotropic AAV...
Inceptor Bio and GRIT Bio Announce Strategic Partnership to Advance IB-T101, a Next-Generation Solid Tumor CAR-T Utilizing the OUTLAST™ Platform
SHANGHAI and MORRISVILLE, N.C., Dec. 18, 2024 /PRNewswire/ -- Inceptor Bio, a leading innovator in cell therapy, and GRIT Bio, a clinical-stage immunotherapy developer, today announced a strategic partnership to advance IB-T101, a potentially best-in-class CAR-T...
Proof-of-concept study bioengineers therapeutics for improved cancer treatment
Credit: Pixabay/CC0 Public DomainA team of Children's Medical Research Institute (CMRI) scientists has identified a new method for producing a therapeutic product that has the potential to improve the treatment of cancer. The work by Associate Professor Leszek...
Related Services

Custom mRNA-LNP Services
READ MORE

Off-the-shelf mRNA
READ MORE

