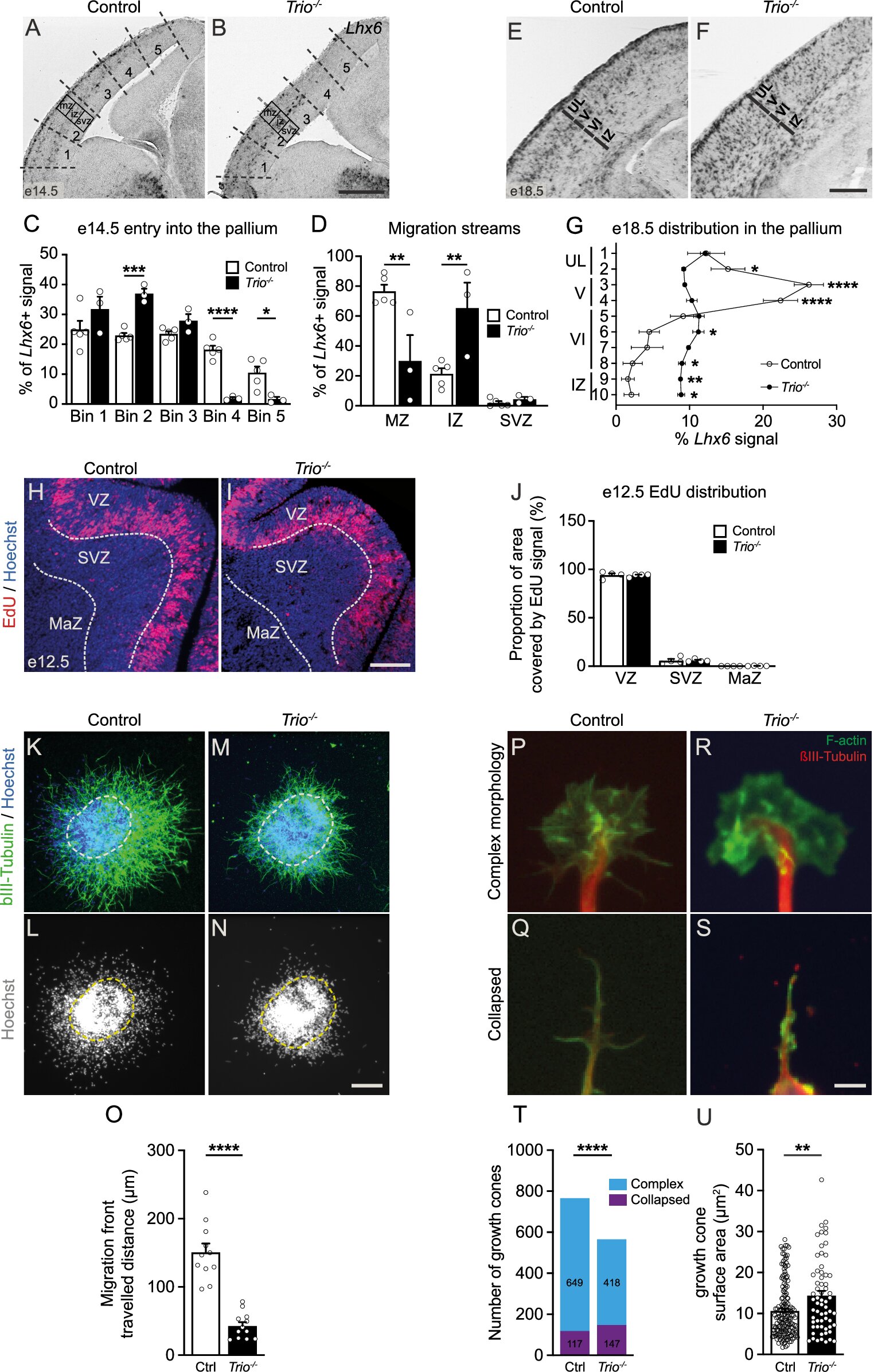
Defective migration of MGE-derived cortical INs in Trio–/– embryos. Credit: Molecular Psychiatry (2024). DOI: 10.1038/s41380-024-02742-y
What are the molecular and cellular mechanisms by which some babies develop epileptic encephalopathies and autism spectrum disorder? That’s what researchers in Canada and France set out to uncover—and they think they’ve found an answer.
It turns out that many of these neurodevelopmental disorders are due to a dysfunction in the development of GABAergic interneurons, cells that are scarce in the brain, but whose inhibitory role is crucial to the proper wiring of the cerebral cortex.
In particular, mutations in the TRIO gene disrupt the migration of these interneurons, impairing their ability to integrate properly into circuits and hindering brain development. That’s the finding of a new study by Université de Montréal postdoctoral fellow Lara Eid led by Dr. Elsa Rossignol at the UdeM-affiliated CHU Sainte-Justine, with Dr. Evelyne Bloch-Gallego of Institut Cochin in Paris.
The study appears in Molecular Psychiatry.
A unique technique
After developing a unique microscopy technique that enables real-time tracking of interneuron movement during the embryonic period, the researchers demonstrated that loss of function of the TRIO gene causes significant difficulties in interneuron migration, a key process in the formation of the cerebral cortex.
During normal brain development in an unborn baby, interneurons must leave a deep embryonic structure and travel long distances to reach the developing cortex.
“What we observe in mouse models with interneuron-specific TRIO deletion is that the affected cells move erratically and more slowly during the embryonic development, as if they don’t know where to go,” said Eid. “This prevents them from reaching their destination, so by the end of the migration, many areas of the cortex don’t have enough of these interneurons to properly exert their inhibitory role.
“What we’re seeing is that after birth, these models develop a range of cognitive and behavioral disorders not unlike those seen in children with epileptic encephalopathy.”
Triggering morphological changes
The study shows that TRIO is a key player in integrating signals from the environment and triggering the morphological changes required for migrating interneurons to orient themselves and move in the right direction and reach their final destination. These migration difficulties therefore reflect dysfunction at two levels: in the neurons’ ability to change shape in order to reorient and propel themselves (intrinsic mechanisms) and in their response to external signals (extrinsic mechanisms).
On the one hand, loss of TRIO function alters interneuron morphology and reduces migration speed. On the other hand, this loss means that interneurons don’t respond well to attractive or guidance signals secreted by surrounding cells or to certain signals that activate the dynamic migration process.
The new study confirms that these two aspects are absolutely necessary for the migration process to proceed normally and that TRIO plays a key role in the regulation of cell mechanics through small molecules called Rho GTPases.
Although research has not yet reached the stage of developing a treatment for TRIO-associated epileptic encephalopathy, the study offers promising avenues for the development of pharmacological treatment, gene therapy and cell therapy involving transplantation of GABAergic interneuron progenitor cells.
“Our results suggest that children with TRIO–associated epileptic encephalopathy could benefit from targeted therapy or cell therapy aimed at restoring the number or function of interneurons,” said Rossignol, co-principal investigator of the study. “This data brings us a step closer to finding a treatment that will improve the outcome for affected children.”

Check out our AAV CDMO service to expedite your gene therapy research
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
The Biggest Medical Advances of 2024: Transforming Lives
The rapid pace of medical innovation continues to redefine healthcare. In 2024, remarkable breakthroughs—from cancer therapies to treatments for rare diseases—have not only improved lives but also shaped the future of medicine. Advancements in Cancer Treatment...
Pfizer ends work on Sangamo’s hemophilia gene therapy, crushing biotech’s hopes
Despite reporting a Phase 3 win this summer, Pfizer terminated its hemophilia A gene therapy pact with Sangamo Therapeutics in a move that cast a pall on the California biotech’s future. In response to Monday’s news about giroctocogene fitelparvovec, Sangamo’s shares...
A Look at 2024’s Patent Expirations and Generic Competition
The pharmaceutical industry faced a major shift in 2024 as several blockbuster drugs lost patent protection. This change brought challenges and opportunities for companies and patients. Pharmaceutical firms experienced revenue declines and tougher competition from...
Precigen Completes Submission of BLA with Request for Priority Review to the FDA for PRGN-2012 for the Treatment of Adults with Recurrent Respiratory Papillomatosis
– PRGN-2012 has the potential to be the first FDA-approved therapeutic for the treatment of adults with RRP, a rare and devastating chronic disease for which the current standard-of-care is repeated surgeries –– PRGN-2012 received Breakthrough Therapy Designation from...
Related Services

AAV Packaging Services
READ MORE

Off-the-Shelf AAV Products
READ MORE

