Revolutionizing CAR T Cell Therapy with Lipid Nanoparticles
Chimeric antigen receptor (CAR) T cell therapy has transformed cancer treatment by turning a patient’s own T cells into powerful cancer-fighting agents. However, as the technology advances, there is an increasing need for more sophisticated genetic modifications. The latest breakthrough involves using lipid nanoparticles (LNPs) to deliver CRISPR-Cas9 genome editing tools to human primary T cells, a method that could enhance CAR T cell therapies and overcome current limitations.
Challenges with Current T Cell Engineering Methods
Traditional methods of T cell engineering involve using viral vectors or electroporation to deliver genetic material into cells. While effective, these approaches have notable drawbacks. Viral vectors can provoke immune responses, have limited capacity, and are costly to produce. Electroporation, on the other hand, uses electrical pulses to introduce genetic material into cells but can compromise cell viability, especially during complex, multi-step engineering processes.
Why Lipid Nanoparticles?
LNPs offer a promising alternative for T cell engineering. These synthetic particles encapsulate and protect RNA, delivering it into cells in a way that resembles the natural uptake of low-density lipoproteins (LDL). This gentle and efficient method avoids the harsh conditions of electroporation, maintaining high cell viability while enabling complex gene editing and protein expression.
The GenVoy-ILM™ T Cell Kit for mRNA: A Game Changer
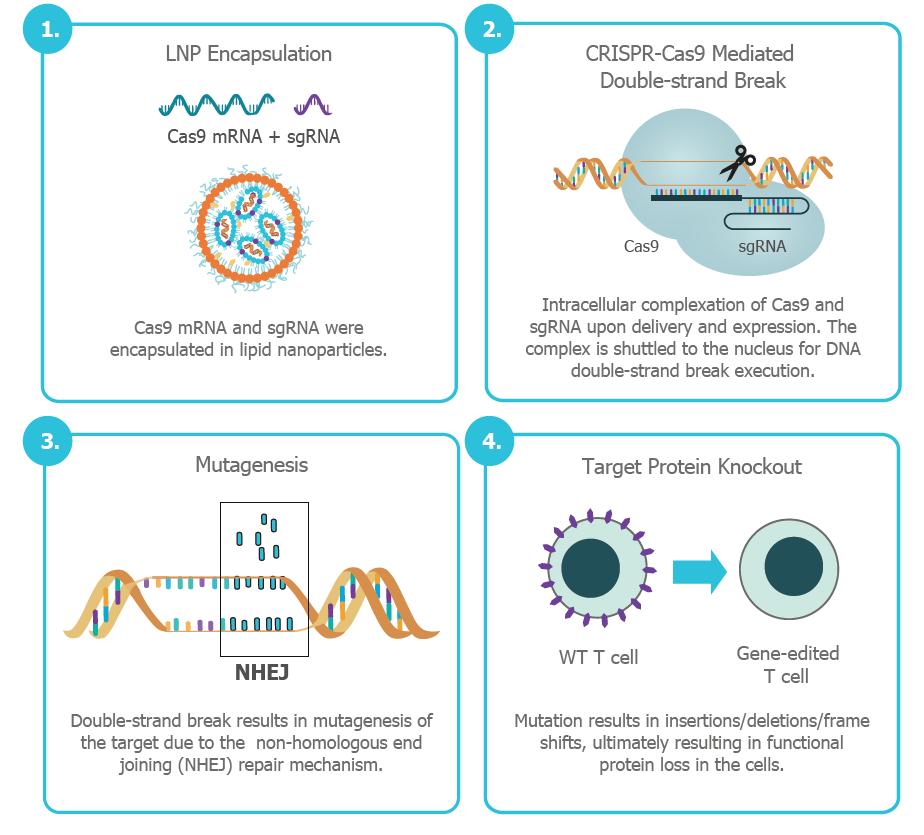
In a recent study, researchers showcased a novel method using the GenVoy-ILM™ T Cell Kit for mRNA to edit T cells via LNPs. The team demonstrated the sequential delivery of Cas9 mRNA and single-guide RNA (sgRNA) to knock out the T cell receptor (TCRαβ), a step toward creating universal CAR T cells from allogeneic donors. This multi-step approach also included introducing CAR mRNA to generate CAR T cells capable of targeting cancer cells.
How It Works: LNP-Mediated CRISPR-Cas9 Editing
LNPs encapsulate Cas9 mRNA and sgRNA, which guide the Cas9 protein to the target DNA within T cells, inducing double-strand breaks that are repaired by the cell, often resulting in gene knockouts. This process disrupts inhibitory pathways exploited by the tumor microenvironment, enhancing the effectiveness of CAR T cells. The researchers achieved a knockout efficiency of 80% with high cell viability, outperforming traditional methods.
Potential Impact on CAR T Cell Therapy
Using LNPs for T cell engineering could significantly enhance the production of CAR T cells by improving cell yield and viability, reducing manufacturing costs, and streamlining the production process. This method is also scalable, making it suitable for clinical applications. The study highlighted that LNP-engineered CAR T cells maintained their therapeutic potential, effectively killing cancer cells in co-culture assays.
A Step Towards the Future of Cell Therapies
The success of LNPs in genome editing and protein expression, as demonstrated in this study, represents a significant advancement in T cell engineering. By leveraging a clinically relevant, scalable method that maintains cell viability and functionality, LNPs could accelerate the development of next-generation CAR T cell therapies. This approach not only addresses current challenges but also sets the stage for more complex and personalized cell-based treatments in the future.
As the field of cell and gene therapy continues to evolve, innovative delivery systems like LNPs will be crucial in overcoming the limitations of existing technologies. With the potential to make gene editing safer, more efficient, and more accessible, LNPs are poised to play a transformative role in the future of cancer treatment and beyond.
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
Vironexis Biotherapeutics Launches with FDA Clearance for First AAV-Delivered Cancer Immunotherapy Trial
SAN DIEGO, Calif., September 12, 2024 – Vironexis Biotherapeutics has launched from stealth, announcing FDA clearance of its IND application for VNX-101, a gene therapy targeting CD19+ acute lymphoblastic leukemia. This marks the first-ever clinical trial of an...
AAV Gene Therapy Revolutionizes Treatment for LCA1, Improving Vision Significantly
Innovative AAV Gene Therapy Dramatically Restores Vision in LCA1 Researchers have made a significant breakthrough in treating Leber congenital amaurosis type 1 (LCA1), a severe inherited retinal disease that causes early childhood blindness, by using an innovative...
Cellular Origins & 3P Innovation Partner to Streamline Cell and Gene Therapy Manufacturing
Cellular Origins, a TTP Company focused on enabling scalable, cost-effective, and efficient manufacture of cell and gene therapies (CGTs), has partnered with 3P innovation, an engineering company and supplier of automated fill-finish equipment, to integrate 3P...
Voyager Therapeutics Expands Gene Therapy Portfolio with Novartis License for Next-Generation Capsid
Voyager Therapeutics, Inc. (Nasdaq: VYGR), a biotechnology company focused on neurogenetic medicines, has entered into a licensing agreement with Novartis AG (NYSE: NVS) for a novel capsid developed using Voyager’s TRACER™ capsid discovery platform. This license will...
Related Services

Custom mRNA-LNP Services
READ MORE

