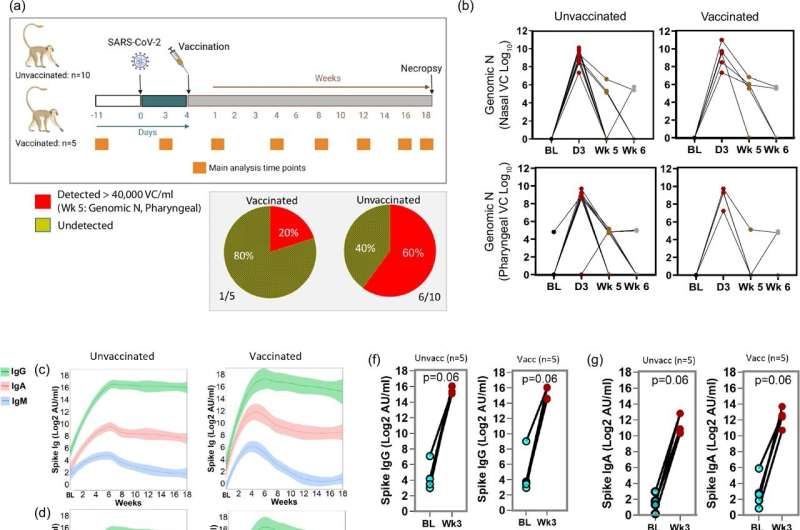
Researchers at Tulane University have discovered a new approach to tackling a lingering health challenge faced by some with long-term COVID: high blood sugar levels.
A new study published in Nature Communications and conducted at the Tulane National Primate Research Center suggests that the COVID-19 vaccine could be used therapeutically to address metabolic complications experienced among those with long-term COVID, sometimes called long-haulers, even if the vaccine is administered several days after infection.
Many people who recover from COVID-19 continue to suffer from various long-term health problems, collectively known as long COVID. One of the most concerning issues is persistent hyperglycemia, or high blood sugar, which can lead to serious health complications such as diabetes and heart disease.
Understanding and addressing this problem has been a major challenge for researchers and health care providers.
To better understand and combat these long-term effects, researchers at the Tulane National Primate Research Center turned to a nonhuman primate model of long-COVID and determined that the model is also appropriate for studying COVID-onset hyperglycemia and diabetes.
The study found that administering the COVID-19 mRNA vaccine four days after infection showed a significant positive effect on blood sugar levels that was sustained over time. This finding suggests that the vaccine could potentially be used not just for prevention but also as a treatment for managing long-term metabolic complications of COVID-19.
Researchers also identified certain inflammatory molecules in the bloodstream linked to high blood sugar levels. The elevated blood sugar seems to result from changes in how the liver stores glucose, even though the virus was no longer present in the liver and pancreas. These findings suggest that diabetes may develop through new mechanisms involving viral infections and inflammation.
“This research opens up a new frontier in our fight against COVID-19,” said Clovis Palmer, Ph.D., one of the lead authors of the study. “By showing that the vaccine can have therapeutic benefits even after infection, we can explore new strategies to help those suffering from long COVID, especially those with symptoms like chronic fatigue that may be linked to metabolic dysfunction.”
Jay Rappaport, Ph.D., co-corresponding author and director of the Tulane National Primate Research Center added, “The discovery that COVID can induce diabetes in an animal model is a significant advancement in our understanding of the long-term effects of COVID. The fact that a COVID vaccine given after infection can have protective effects highlights the importance of innovative research in addressing the ongoing challenges of a pandemic.”
https://medicalxpress.com/news/2024-08-vaccine-high-blood-sugar-covid.html

Check out our mRNA service to expedite your vaccine research
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
Related News
[2024/11/22] Gene and Cell Therapy- weekly digest from PackGene
FeaturedNewsArticlesPackGene's NewsletterReceive the latest news and insights to your inbox.About PackGenePackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span...
Novartis seeks more bolt-on deals as it purchases neuro startup for up to $1.1B
Novartis is buying gene therapy and neuroscience biotech Kate Therapeutics in a deal worth $1.1 billion in upfront and milestone payments, the Swiss pharma confirmed to Endpoints News on Thursday. And Novartis is not done yet. It is still on the lookout for bolt-on...
Genprex Signs Exclusive License to Additional Gene Therapy Technologies with the University of Michigan for the Treatment of Lung Cancer
License includes Genprex's Reqorsa® Gene Therapy in Combination with ALK-Inhibitors for the Potential Treatment of ALK-Positive Lung Cancer AUSTIN, Texas, Nov. 20, 2024 /PRNewswire/ -- Genprex, Inc. ("Genprex" or the "Company") (NASDAQ: GNPX), a clinical-stage...
Vyriad announces strategic collaboration with Novartis to develop in vivo CAR-T cell therapies
Collaboration will combine Vyriad's lentiviral vector platform and Novartis expertise and leadership in cell therapy innovation ROCHESTER, Minn., Nov. 20, 2024 /PRNewswire/ -- Vyriad, Inc., a clinical-stage biotechnology company developing the next generation of...
Related Services

AAV Packaging Services
READ MORE

Off-the-Shelf AAV Products
READ MORE

