The incidence of ALS is typically 1.5-2.4 per 100,000 people, with the age of onset mostly between 40-70 years, and an average onset age of 55. Most ALS patients die within 3-5 years after diagnosis. Although ALS is a rare disease, it is not far from us. Notable patients include renowned astrophysicist Stephen Hawking, former Vice President of JD.com Cai Lei.
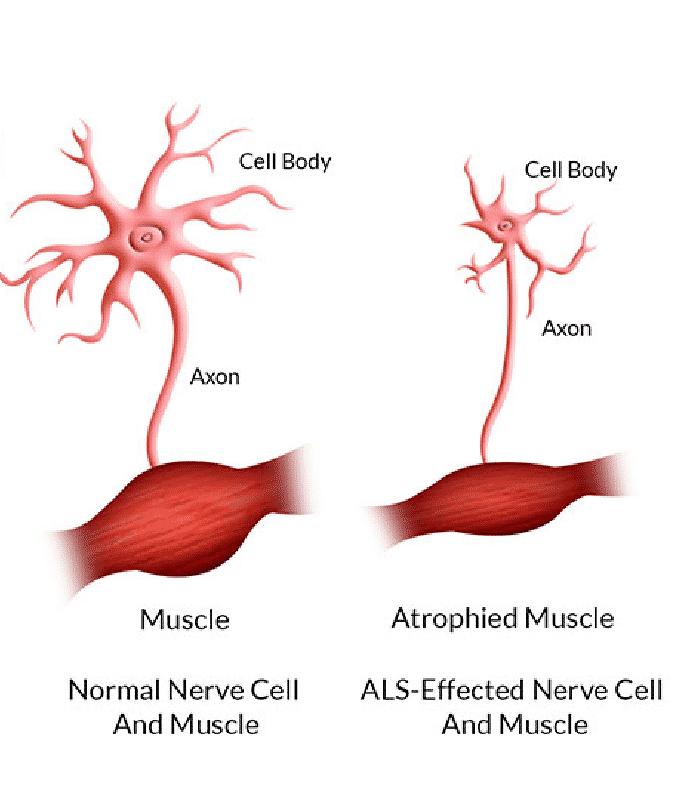
Unclear Pathogenesis, Only Four FDA-Approved Drugs
The pathogenesis of ALS is not entirely clear, with various factors, including genetic and environmental, considered as potential causes. Only 5%-10% of cases have a clear genetic factor, while the remaining 90%-95% are sporadic. Environmental factors such as air pollution, consumption of mercury-contaminated seafood, and pesticide-laden agricultural products increase the risk of developing ALS.
Currently, ALS remains incurable, and treatment focuses on alleviating symptoms and improving quality of life through medication and good care. To date, only four drugs have been approved by the FDA for ALS treatment: the chemical drugs Riluzole, Edaravone, Relyvrio, and the gene therapy drug Tofersen. Tofersen is approved for treating ALS patients with superoxide dismutase 1 (SOD1) mutations based on reduced plasma neurofilament light chain (NfL) levels. It costs $14,230 per injection (approximately RMB 102,000 per injection) and is administered intrathecally, requiring 14 injections in the first year (about RMB 1.43 million per year), and 13 injections annually thereafter to maintain the dose (about RMB 1.33 million per year).
Gene Therapy Brings New Hope
In recent years, with significant progress in basic research, gene therapy has become a promising approach. Many companies domestically and internationally have made strides in this field. In November 2023, the ALS gene therapy drug SNUG01 by Shenjing Changhua, developed by Professor Fan Dongsheng’s team at Peking University Third Hospital, completed the first subject administration in an Investigator-Initiated Trial (IIT) in China, marking a breakthrough in domestic ALS gene therapy.
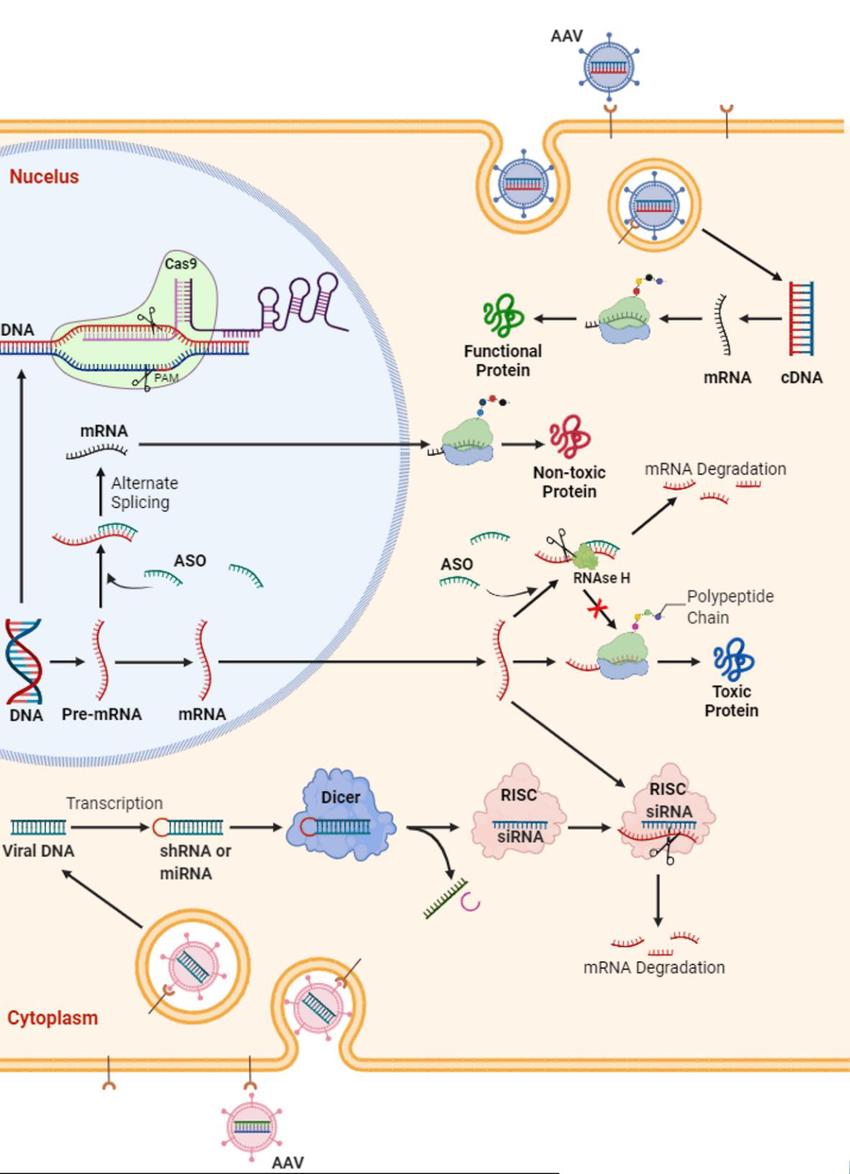
Current gene therapy strategies for ALS primarily include: (i) using microRNA or antisense oligonucleotides (ASO) to eliminate or suppress abnormal transcripts at the RNA transcription level; (ii) using RNA interference (RNAi) to degrade abnormal mRNA; (iii) using methods like CRISPR/Cas for DNA genome editing. The marketed ALS gene therapy drug Tofersen adopts the first strategy, with other strategies being tested in clinical trials for ALS.
Searches on Clinicaltrials and the Chinese Clinical Trial Registry indicate a yearly increase in ALS gene therapy clinical trials, focusing mainly on microRNA or ASO approaches, targeting SOD1, C9ORF72, and FUS. Most clinical trials are still in early stages. From clinical research results, ALS gene therapy drugs have shown good safety and some efficacy, but further clinical trials and research are needed to optimize them for best therapeutic outcomes.
| Agent | Mechanism of Action | Primary Measure Outcomes | Trial Design | N | Sites of Study | Status | CTI | Primary Outcome |
|---|---|---|---|---|---|---|---|---|
| BIIB067 or Tofersen (VALOR Trial) | ASO against SOD1 mRNA | Safety, tolerability, pharmacokinetics, biomarkers, ALSFRS-R change at 28 weeks | Phase 3, randomized, quadruple-blinded, | 183 | USA, Canada, Europe | Complete | NCT-02623699 | N/A |
| placebo-controlled | ||||||||
| AE and SAE up to 248 weeks | Extension of Phase 3, placebo-cotrolled, open label | 183 | USA, Canada, Europe | Active | NCT-03070119 | N/A | ||
| ISIS 333611 [33,34] | ASO against SOD1 mRNA | Safety, tolerability, and pharmacokinetics at unknown time | Phase 1, quadruple-blinded, randomized, placebo-controlled | 33 | USA | Complete | NCT-01041222 | No AE, Well tolerated, dose-dependent CSF and plasma concentrations |
| [37] | AAV-miR-SOD1 | Safety, tolerability, and pharmacokinetics | Open-label | 2 | USA | Complete | N/A | Meningoradiculitis in case 1, but not in case 2 with immunosuppressive therapy; Transient improvement in muscle sctregnth in case 1; |
| BIIB078 | ASO against C9orf72 mRNA | Safety at 323 days | Phase 1, quadruple-blinded, randomized, placebo-controlled | 90 | USA, Canada, Europe | Complete | NCT-03626012 | N/A |
| SB-509 [38] | Plasmid encoding a zinc finger DNA-binding protein transcription factor (ZFP TF(TM)) designed to up-regulate the expression of the gene encoding vascular endothelial growth factor (VEGF-A) | Change in ALSFRS-R at 11 months | Phase 2, open label | 45 | USA | Complete | NCT-00748501 | Safe, delayed deterioration in ankle and toe strength in 40% of treated subjects |
| ION363 (Jacifusen) | ASO against FUS mRNA | Change in ALSFRS-R and Ventilation Assistance-free survival (VAFS) at 505 days | Phase 1–3, double-blinded, randomized, placebo-controlled | 77 | USA, Canada, Belgium, UK | Active | NCT-04768972 | N/A |
Chart 1: Summary of Global Clinical Trials for ALS Gene Therapy Drugs
Summary and Outlook
While ALS treatment has made steady progress, there is still an urgent need to develop more effective therapies to prevent neurodegeneration and alleviate symptoms. The expectations for ALS gene therapy remain high. The key to developing ALS gene therapy drugs lies in understanding ALS causative genes, susceptibility genes, and disease-modifying genes. As a rare disease, ALS has a small patient population, making it challenging to obtain clinical samples, which hinders research progress. Additionally, the construction of disease animal models, difficulty in recruiting clinical trial subjects, and high drug development costs are limiting factors. Fortunately, with concerted efforts in recent years, ALS has gained widespread attention, and domestic ALS gene therapy drug development is accelerating.
In the future, PackGene will continue to uphold the mission of “making gene therapy affordable for the public,” maintaining a customer-oriented approach, and deepening the development of vector processes and platform technologies. We will also keep track of ALS gene therapy drug development, providing one-stop CMC solutions for cell and gene therapy drug clients, including druggability assessment, process development, analytical method development, pilot production, testing and release, US and China registration, and commercial production. This will advance the development and clinical translation of cell and gene therapy drugs, facilitating their regulatory approval.
References
1. Wang, H., L. Guan and M. Deng, Recent progress of the genetics of amyotrophic lateral sclerosis and challenges of gene therapy. Front Neurosci, 2023. 17: p. 1170996.
2. Fang, T., et al., Gene Therapy in Amyotrophic Lateral Sclerosis. Cells, 2022. 11(13).
3. Seelen, M., et al., Long-Term Air Pollution Exposure and Amyotrophic Lateral Sclerosis in Netherlands: A Population-based Case-control Study. Environ Health Perspect, 2017. 125(9): p. 097023.
4. Clinicaltrials.gov
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
More Articles
Advancing AAV-Based Gene Therapy for Hearing Loss Using Mini-PCDH15 Variants
Hearing loss affects millions of people worldwide and can be caused by genetic defects in key proteins essential for auditory function. Recent research by Pedro De-la-Torre and colleagues (doi: https://doi.org/10.1101/2024.06.16.599132) has provided significant...
AAV Vectors in Cancer Therapy: A Review of Applications and Strategies
1. Introduction Cancer continues to be a major health concern despite progress in traditional treatments like surgery, chemotherapy, and radiotherapy. Gene therapy provides an innovative approach by introducing therapeutic genes to cancer cells, enabling targeted...
Advances in AAV-SB Transposon Hybrid Systems for Liver-Targeted Gene Therapies
*Nicolás Sandoval-Villegas, Zoltán Ivics, The best of both worlds: AAV-mediated gene transfer empowered by LNP delivery of Sleeping Beauty transposase for durable transgene expression in vivo, Molecular Therapy, Volume 32, Issue 10, 2024, Pages 3211-3214, ISSN...
Novel Approach in T Cell Engineering: Lipid Nanoparticles Enable Advanced Genome Editing for Cancer Therapies
Revolutionizing CAR T Cell Therapy with Lipid Nanoparticles Chimeric antigen receptor (CAR) T cell therapy has transformed cancer treatment by turning a patient’s own T cells into powerful cancer-fighting agents. However, as the technology advances, there is an...
Related Services
AAV Packaging Services
READ MORE
AAV Packaging Service (NHP)
READ MORE
AAV Packaging Service (HT)
READ MORE

