In pharmacological treatments for glaucoma, the primary mechanisms for lowering IOP include inhibiting aqueous humor secretion or facilitating aqueous humor drainage. Carbonic anhydrase inhibitors (CAIs) are among the oldest IOP-lowering drugs, reducing aqueous humor production by inhibiting the interconversion of carbon dioxide and bicarbonate in the ciliary body. However, topical drug treatments can be complicated by poor patient compliance, low bioavailability, and side effects. Given that glaucoma requires lifelong management as a chronic disease, daily medication and combination therapy with multiple drugs are common in clinical practice. Thus, developing a convenient, effective, and durable treatment method is urgently needed for the long-term management of glaucoma patients. Due to the eye’s accessible nature and immune-privileged characteristics, gene therapy is considered a promising new approach for glaucoma treatment.
On April 25, 2024, the team of Xiulan Zhang from the Zhongshan Ophthalmic Center at Sun Yat-sen University, and the team of Patrick Yu Wai Man from the University of Cambridge jointly published a research paper titled “CRISPR-Cas9-mediated deletion of carbonic anhydrase 2 in the ciliary body to treat glaucoma” in “Cell Reports Medicine” (IF 14.3). Dr. Jiaxuan Jiang and Dr. Kangjie Kong are the co-first authors of this paper. The study used the AAV ShH10 to deliver the CRISPR-Cas9 system, precisely targeting the Car2 gene in the ciliary body. This approach effectively reduced IOP in an experimental glaucoma mouse model and provided long-term mitigation or even prevention of glaucoma damage caused by sustained high IOP, offering a potential new treatment strategy for glaucoma patients.
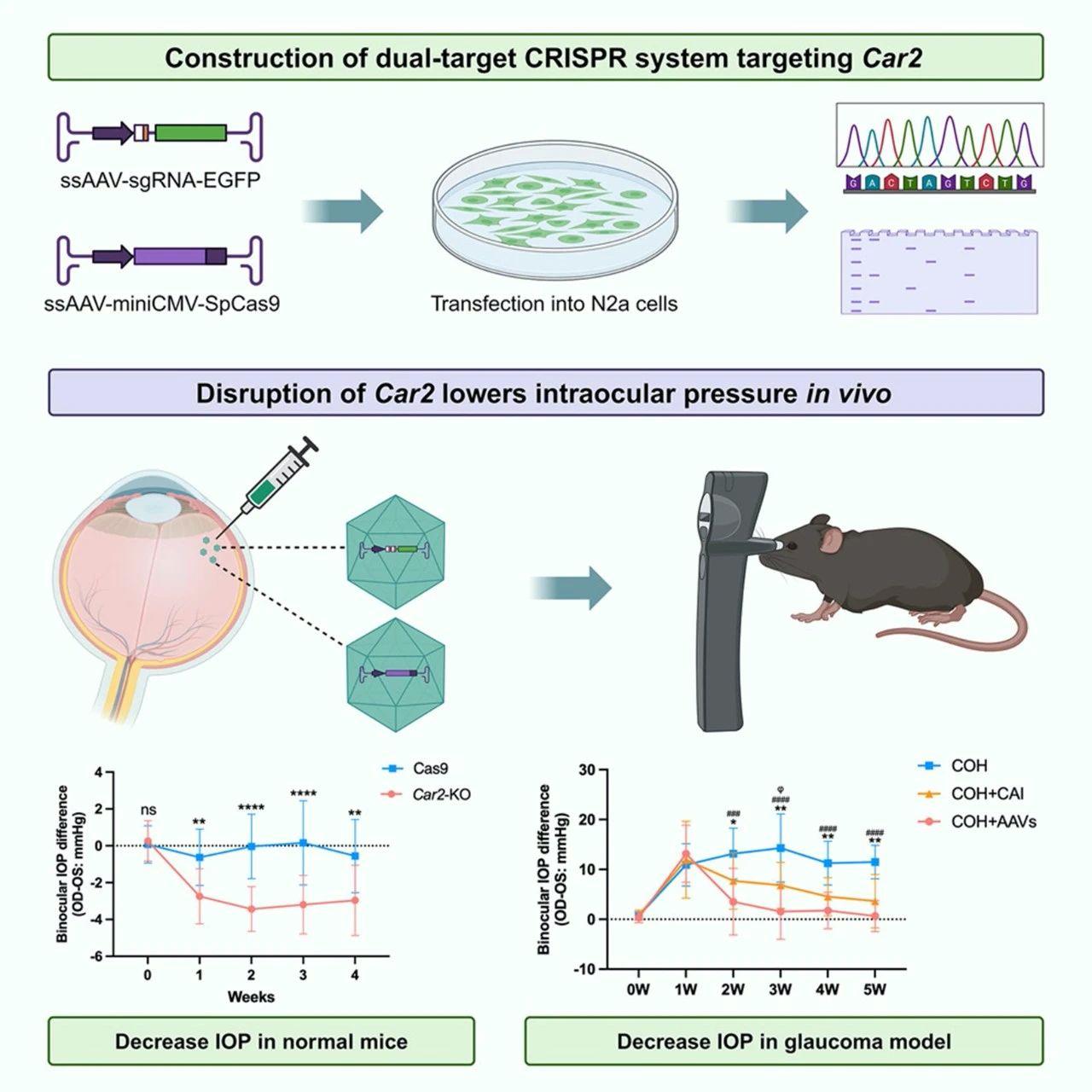
*PackGene Contributed the vector construction and AAV packaging services for this research
In Vitro Experiments
- Plasmid Vectors: ssAAV.U6.(Sp)sgRNA.CAG.SV40 NLS-EGFP.WPRE, ssAAV.miniCMV.SpCas9
- Promoters: U6, miniCMV, CAG
- Cell Type: Neuro-2a (N2a) cells
- Transfection Method: Lipofectamine 3000 transfection
- Detection Methods: Sanger sequencing, T7E1 assay, GUIDE-seq
In Vivo Experiments
- Viral Vectors: AAV shH10-SpCas9, AAV shH10-sgRNA
- Promoters: U6, miniCMV, CAG
- Injection Sites: DBA/2J mice, intravitreal injection
- Injection Dose: 1 x 10^13 gc/mL, 2μL
- Detection Time: 1 week post-injection
- Detection Methods: Immunofluorescence imaging, Capillary-based immunoassay, qPCR, etc.

Check out our AAV CDMO service to expedite your gene therapy research
1. DOI: https://doi.org/10.1016/S0140-6736(17)31469-1
2. DOI: https://doi.org/10.1016/j.xcrm.2024.101524
PackGene Biotech is a world-leading CRO and CDMO, excelling in AAV vectors, mRNA, plasmid DNA, and lentiviral vector solutions. Our comprehensive offerings span from vector design and construction to AAV, lentivirus, and mRNA services. With a sharp focus on early-stage drug discovery, preclinical development, and cell and gene therapy trials, we deliver cost-effective, dependable, and scalable production solutions. Leveraging our groundbreaking π-alpha 293 AAV high-yield platform, we amplify AAV production by up to 10-fold, yielding up to 1e+17vg per batch to meet diverse commercial and clinical project needs. Moreover, our tailored mRNA and LNP products and services cater to every stage of drug and vaccine development, from research to GMP production, providing a seamless, end-to-end solution.
More Articles
Advancing AAV-Based Gene Therapy for Hearing Loss Using Mini-PCDH15 Variants
Hearing loss affects millions of people worldwide and can be caused by genetic defects in key proteins essential for auditory function. Recent research by Pedro De-la-Torre and colleagues (doi: https://doi.org/10.1101/2024.06.16.599132) has provided significant...
AAV Vectors in Cancer Therapy: A Review of Applications and Strategies
1. Introduction Cancer continues to be a major health concern despite progress in traditional treatments like surgery, chemotherapy, and radiotherapy. Gene therapy provides an innovative approach by introducing therapeutic genes to cancer cells, enabling targeted...
Advances in AAV-SB Transposon Hybrid Systems for Liver-Targeted Gene Therapies
*Nicolás Sandoval-Villegas, Zoltán Ivics, The best of both worlds: AAV-mediated gene transfer empowered by LNP delivery of Sleeping Beauty transposase for durable transgene expression in vivo, Molecular Therapy, Volume 32, Issue 10, 2024, Pages 3211-3214, ISSN...
Novel Approach in T Cell Engineering: Lipid Nanoparticles Enable Advanced Genome Editing for Cancer Therapies
Revolutionizing CAR T Cell Therapy with Lipid Nanoparticles Chimeric antigen receptor (CAR) T cell therapy has transformed cancer treatment by turning a patient’s own T cells into powerful cancer-fighting agents. However, as the technology advances, there is an...
Related Services
AAV Packaging Services
READ MORE
AAV Packaging Service (NHP)
READ MORE
AAV Packaging Service (HT)
READ MORE

