These assessments collectively offer a comprehensive evaluation of the AAV vectors, ensuring that your AAVs meet even the most stringent standards for safe and effective gene therapy applications.
Extensive Capabilities
Safety Assurance
Timely Results
Expert Guidance
Uncompromising Quality
Tailored Solutions
We offer more than 40 standarized AAV quality assays.
| Category | SKU# | Test | Method | Turnaround Time (Calendar Day) |
| Titration | AAVQC001-001 | AAV Genome Titration <ddPCR> | ddPCR | 5 |
| AAVQC001-002 | Custom ddPCR probe and primer synthesis | – | 5-7 | |
| AAVQC001-003 | AAV Capsid Titration | ELISA | 5 | |
| AAVQC001-004 | Infectious Titer <TCID50> | Serial Dilution and qPCR | 21 | |
| Genome Integrity | AAVQC002-001 | AAV Genomic Integrity <CE> | CE | 5 |
| AAVQC002-002 | AAV Genomic Sequencing <TGS> | 3rd Generation Sequencing | 5-10 | |
| AAVQC002-003 | AAV Identity by Sanger sequencing | Sanger sequencing | 3-5 | |
| AAVQC002-004 | Alkaline Gel Electrophoresis | Agarose gels | 6 | |
| Capsid characterization, purity and aggregation | AAVQC003-001 | AAV Empty Capsid Rate <AUC> | AUC to analyze empty capsid rate | 5 |
| AAVQC003-002 | TEM (5 pictures) +report | TEM | 3-5 | |
| AAVQC003-003 | DLS (Dynamic light scattering) | Dynamic light scattering | 5 | |
| AAVQC003-004 | AAV Capsid Peptide Mapping | HPLC-MS/MS | 21 | |
| AAVQC003-005 | Capsid Protein Molecular Weight and Ratio | RP-HPLC-MS | 21 | |
| AAVQC003-006 | Capsid purity Analysis <SDS-PAGE> | SDS-PAGE with Silver Staining | 2-3 | |
| AAVQC003-007 | Capsid purity Analysis <SDS-PAGE> | SDS-PAGE with Coomassie Blue Staining | 5 | |
| AAVQC003-008 | Capsid purity Analysis <CE-SDS> | CE-SDS | 5 | |
| AAVQC003-009 | Purity Analysis <AEC-HPLC> | AEC-HPLC | 5 | |
| AAVQC003-010 | Purity and Aggregation Analysis <SEC-HPLC> | SEC-HPLC | 5 | |
| Contamination | AAVQC004-001 | Residual Host HEK293 Cell DNA Quantification | qPCR | 5 |
| AAVQC004-002 | Residual HEK293 Host Cell DNA Sizing | qPCR | 5 | |
| AAVQC004-003 | Residual Plasmid DNA | ddPCR | 5 | |
| AAVQC004-004 | Residual E1A | ddPCR | 5 | |
| AAVQC004-005 | Residual Host Cell Protein | ELISA | 5 | |
| AAVQC004-006 | Residual BSA | ELISA | 5 | |
| AAVQC004-007 | Residual Nuclease | ELISA | 5 | |
| AAVQC004-008 | Residual Affinity Ligands | ELISA | 5 | |
| AAVQC004-009 | Residual PEI | HPLC | 5 | |
| AAVQC004-010 | Residual Tween20 | HPLC | 5 | |
| AAVQC004-011 | Residual Triton X100 | HPLC | 5 | |
| AAVQC004-012 | Residual Iodixanol | HPLC | 5 | |
| AAVQC004-013 | Residual Poloxamer 188 | HPLC | 5 | |
| AAVQC004-015 | Residual Triton Analysis(bundle with endo removal) | HPLC | 5 | |
| Safety | AAVQC005-001 | Endotoxin removal | Triton | 2-3 |
| AAVQC005-002 | Sterility test | Innoculation | 14-20 | |
| AAVQC005-003 | Mycoplasma detection | Gel | 2-3 | |
| AAVQC005-004 | Endotoxin test | LAL | 3 | |
| AAVQC005-005 | Quantitative Endotoxin test | Kinetic Chromogenic Assays | 3 | |
| AAVQC005-006 | Bioburden Test | Plate count method | 5 | |
| AAVQC005-007 | Mycoplasma detection by qPCR | qPCR | 2-3 | |
| AAVQC005-008 | Replication Competent AAV (rcAAV) Analysis | Infection on Permissive Cells | 21 |
(Scroll down to see more)
AAV Genome Titer by ddPCR
Provides precise quantification of viral genome copies, ensuring accurate assessment of viral load. This is essential for determining effective dosages and maintaining batch-to-batch consistency in gene therapy applications.
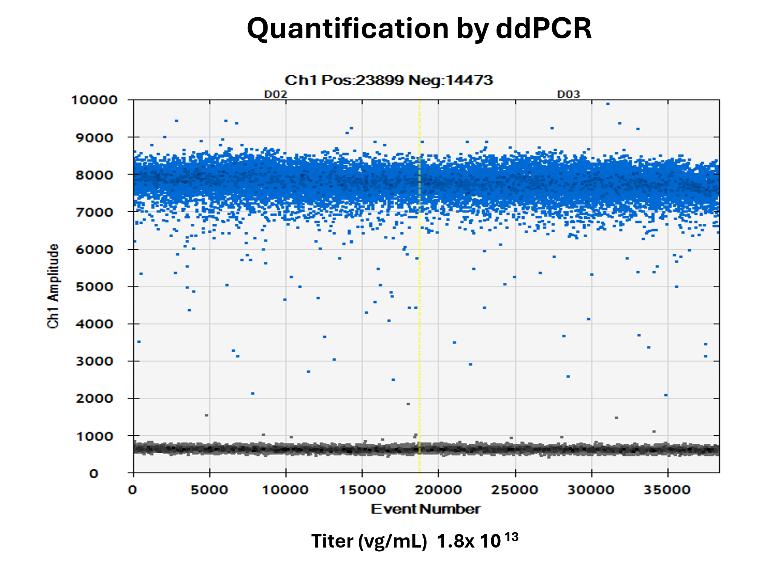 AAV genome titer by ddPCR
AAV genome titer by ddPCR
AAV Genome Integrity by CE
Evaluates the structural integrity of AAV genomes by identifying fragmentation or degradation. These measures are key determinants of AAV stability, efficacy, and safety.
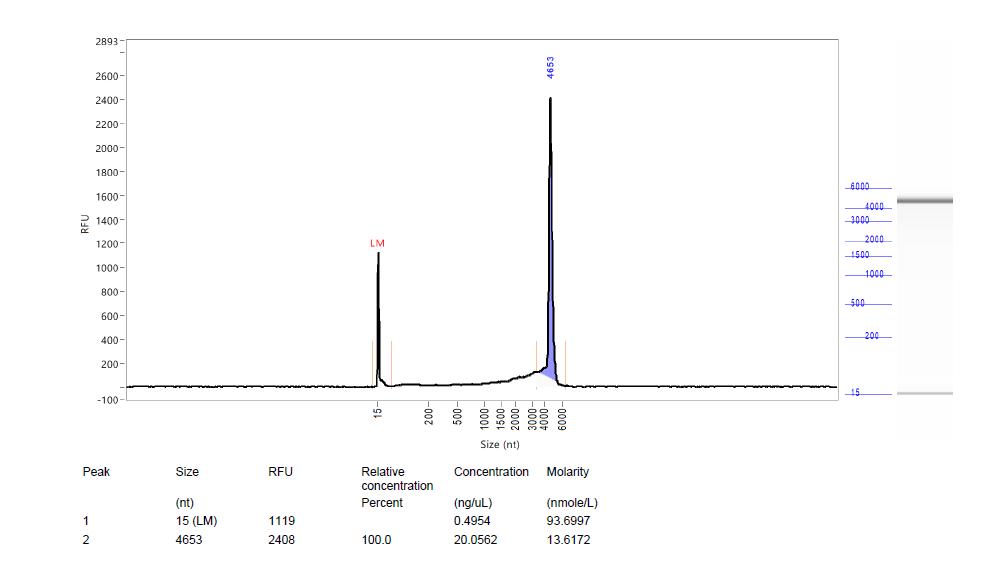 AAV genome integrity analysis by CE
AAV genome integrity analysis by CE
AAV Genome Sequencing by Nanopore
Offers detailed analysis of the AAV genome by detecting mutations, insertions, and deletions. This comprehensive genome evaluation ensures the fidelity and stability of the AAV vector and is essential for therapeutic use.
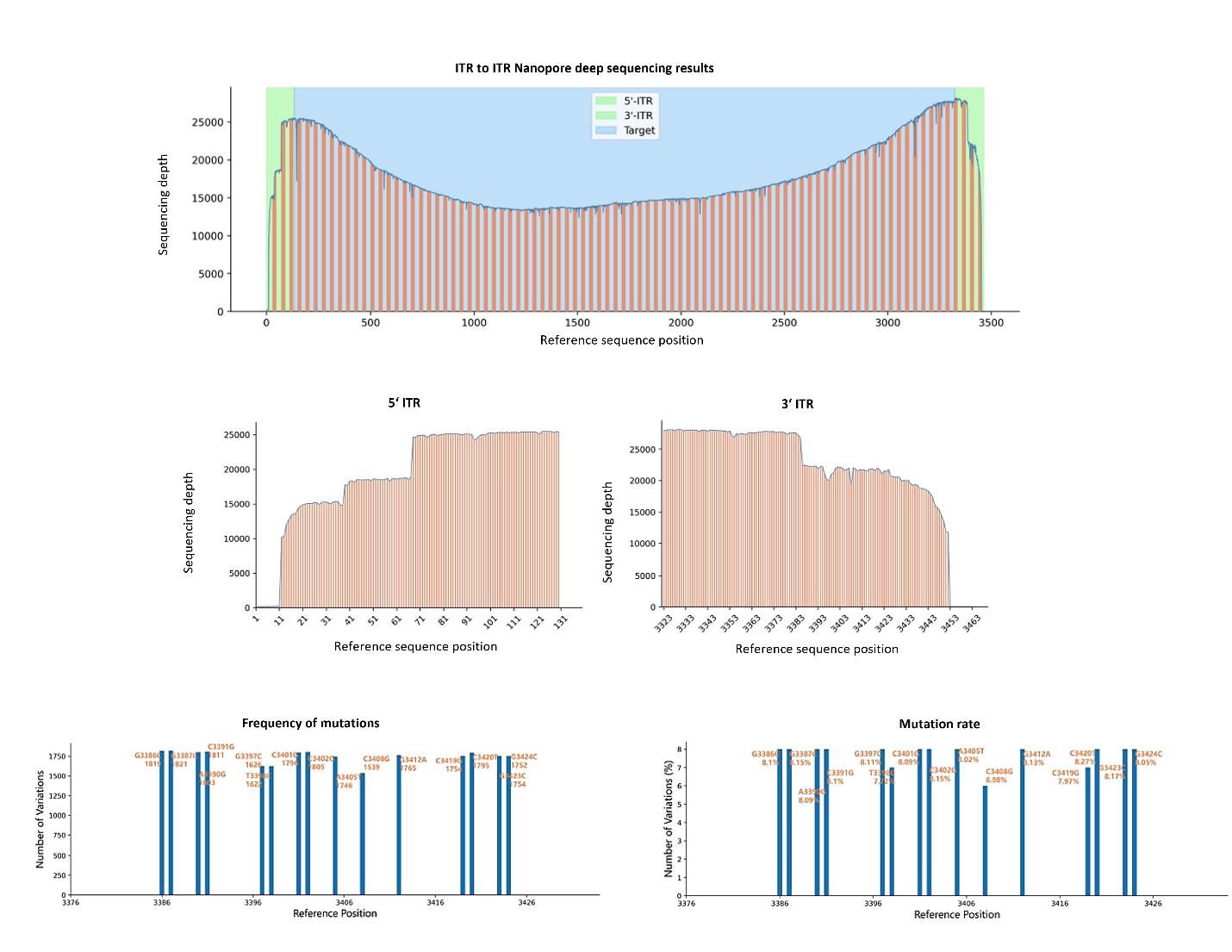 Nanopore sequencing reads depth from 5‘ ITR to 3’ ITR
Nanopore sequencing reads depth from 5‘ ITR to 3’ ITR
AAV Empty Capsid Rate Test by AUC
Measures the proportion of empty versus full capsids, which is critical for determining the potency and immunogenicity of a therapy. AUC is the gold-standard method for measuring empty capsid rate and helps to ensure the delivery of high-quality viral vectors.
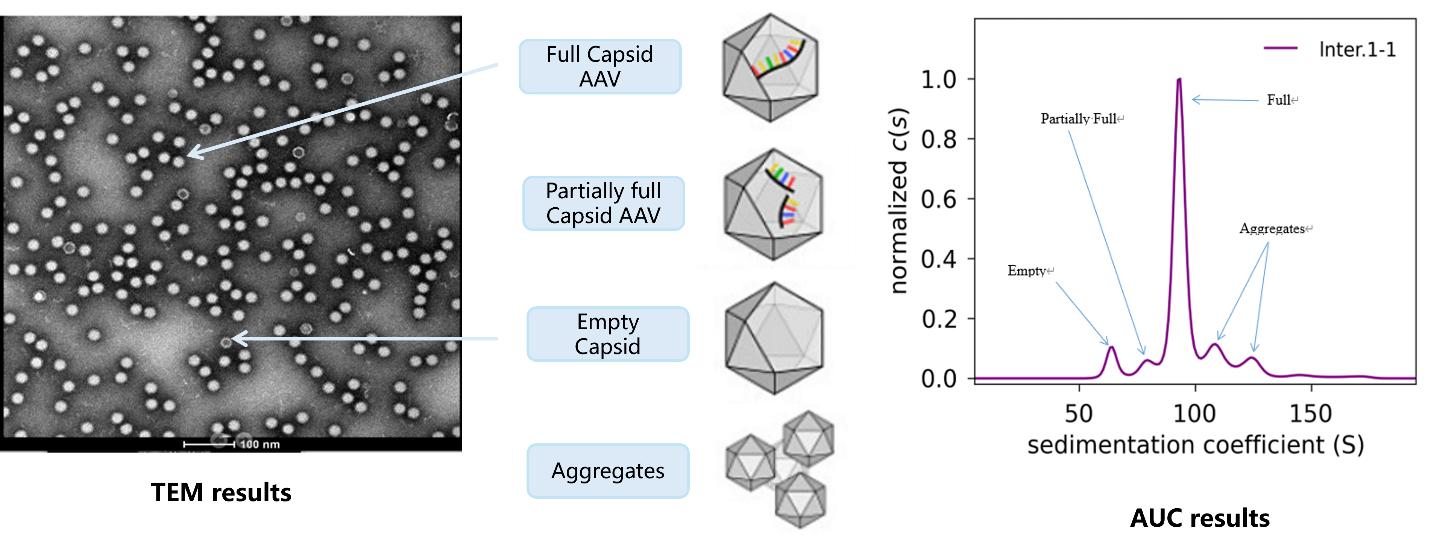 AAV empty capsid rate analysis by AUC
AAV empty capsid rate analysis by AUC
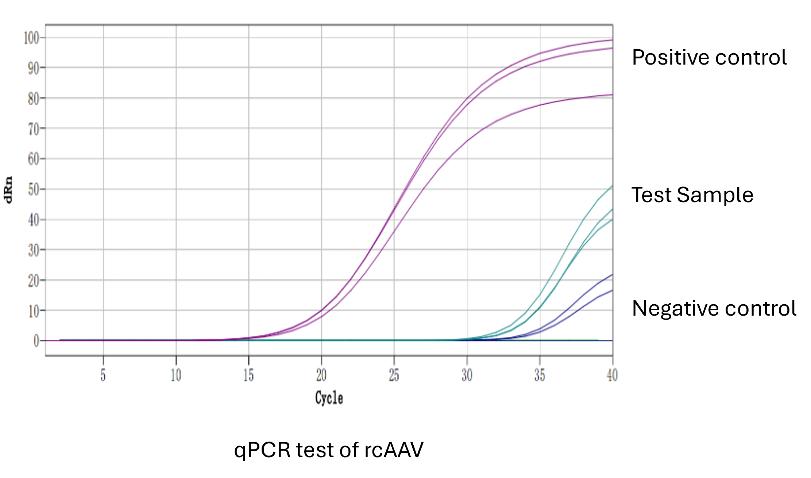
Replication Competent AAV (rcAAV) Analysis
Detects and quantifies replication-competent AAV, which is essential for ensuring the safety of gene therapies. Minimizing rcAAV contamination is necessary to prevent unwanted viral replication in patients.
How much plasmid do I need to provide for AAV packaging
You only need to provide 1-4 µg of plasmid. We will handle the plasmid preparation necessary for AAV packaging. You don't need to purchase an additional plasmid prep service unless you wish to receive more plasmid from us. Please note, the timeline in our quote already includes the plasmid preparation.
What are the difference between research and NHP grade?
Research-grade AAV is actually the most common grade used for research and development, while NHP-grade is where we've improved the purification process and more stringent QC test, resulting in higher purity, lower endotoxin, better genome integrity and lower empty capsid rates. Since animal experiments demand higher virus quality and better consistency, we recommend using our NHP-grade AAV for large animal experiments, such as NHP, porcine, canine, etc. Of course, if you're conducting cell experiments and desire higher purity, that's also recommended.
How do you choose the fluorescent or luminescent marker for live imaging in mice?
For in vivo imaging, it's generally advised to use vectors with luciferase.
Currently, in vivo imaging primarily utilizes two techniques: bioluminescence and fluorescence. Bioluminescence involves using the luciferase gene to label cells or DNA, while fluorescence employs fluorescent proteins such as GFP, EGFP, RFP, YFP, mCherry, etc., to mark cells or proteins. Bioluminescence offers advantages like straightforward operation, sensitive response, rapid imaging, and clear visualization. However, its drawback lies in its relatively weak signal, necessitating the use of CCD lenses for detection and requiring instruments with high precision. On the contrary, fluorescence allows for the utilization of various proteins for labeling and enables multiplex labeling, making the process relatively straightforward. Nevertheless, nonspecific fluorescence imposes limitations on its sensitivity, necessitating the use of excitation lights of different wavelengths, thereby making precise in vivo quantification challenging. Bioluminescence relies on the interaction with luciferase to emit light, demonstrating high specificity. The red light emitted by luciferase penetrates tissues nearly 100 times more effectively in vivo than the green light emitted by green fluorescent protein, resulting in a higher signal-to-noise ratio. While fluorescent proteins necessitate excitation light to produce reflected light, nonspecific fluorescence from the mouse's fur reduces the signal-to-noise ratio during the detection process. Fluorescent protein detection is more suited to ex vivo detection, whereas luciferase detection is better suited to in vivo detection. Currently, luciferase labeling is more commonly employed. There are two frequently used luciferases: Firefly Luciferase (Fluc) and Renilla Luciferase (Rluc), each utilizing different substrates—D-Luciferin for the former and Coelenterazine for the latter. They emit light of varying colors, with the former emitting light at approximately 560nm and the latter emitting light at approximately 450-480nm. The light emitted by the former penetrates tissues more effectively, while the latter undergoes faster metabolism in vivo compared to the former. Typically, the former is utilized as a reporter gene, although both can be simultaneously employed for dual labeling.
What quality control tests do you conduct for your AAV?
Our AAV products are subjected to standard release testing procedures, including endotoxin assessment using Limulus Amebocyte Lysate (LAL) assay, purity analysis via Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE), and titer determination using quantitative Polymerase Chain Reaction (qPCR) or droplet digital PCR(ddPCR). Moreover, we conduct restriction enzyme digestion for the Gene of Interest (GOI) plasmids utilized in packaging. Different grade AAV may include different QC tests as listed here.
In addition, we offer 40+ analytical tests to measure titer, AAV genome integrity, characterization, purity, aggregation, contamination and safty, including TEM, AUC, TCID50, Nanopore deep sequencing and may others. Please refer to our anlytical tests webpage.
How is the titer of AAV determined?
During AAV titer measurement, our instruments are initially calibrated using the AAV standard product ATCC VR-1816™, a globally recognized reference titer verified by 16 laboratories. Subsequently, we employ SYBR Green qPCR methodology to ascertain titers, achieving values of 1E+13GC/ml or higher. This meticulous approach ensures alignment with prevailing academic standards and prevents inaccurate titration results.
Besides qPCR, we have several other methods available for AAV titer determination:
-
- Genome titer detection by ddPCR.
- Capsid titer detection by ELISA technology.
- Infectious titer detection by TCID50.
What are the difference between scAAV and ssAAV?
Adeno-associated viruses (AAV) are single-stranded DNA viruses (ssAAV) that must first undergo a transition from single-stranded genome to transcriptionally active double-stranded form before expression can begin. This process limits gene transduction mediated by AAV vectors and directly affects gene expression efficiency. Self-complementary double-stranded DNA adeno-associated viruses (scAAV), on the other hand, mutate the 3' ITR trs site, forming double-stranded DNA packaged into AAV. They do not require the transition from single-stranded to double-stranded form. In other words, after entering cells, scAAV viruses can express directly and more rapidly, with higher expression levels. The drawback of scAAV is its smaller packaging capacity and the potential to enhance immunogenicity. It is suitable for research requiring faster expression of target genes or stronger expression of genes smaller than 2.2kb.
Which serotypes does rAAV encompass, and how do you determine the suitable serotype?
As of now, nine naturally occuring serotypes of human AAV have been discovered (AAV1/2/3/4/5/6/7/8/9) and widely applied in scientific research. AAV10 and AAV11 were first discovered in non-human primates in 2004, and no cross-reactivity was observed between AAV10, AAV11, and AAV2, making them promising candidate vectors. Subsequently, researchers isolated AAV12 and AAV13 from simian adenovirus, with limited research on these serotypes currently. Based on these wild-type AAVs, researchers have developed many AAV mutants, such as AAV-DJ and the PHP series, through various modification strategies.
Due to differences in the spatial structure of capsid proteins among AAV serotypes, there are significant variations in their recognition and binding to cell surface receptors, leading to tropism of different AAV serotypes for different tissues. When selecting serotypes, experimental purposes can refer to AAV serotypes used in peer-reviewed literature. For example, AAV1 and AAV9 are more commonly used in brain research than other wild-type AAV serotypes, while AAV6 exhibits higher lymphocyte selectivity.
There are also many engineered serotypes that have been modified or engineered to enhance specific properties for gene therapy applications. These modifications can include alterations to the capsid proteins to change tissue tropism, improve transduction efficiency, evade immune responses, or increase payload capacity. Engineered AAV serotypes have been developed through various strategies such as directed evolution, rational design, or hybridization of existing serotypes. These engineered serotypes offer enhanced performance and versatility, making them valuable tools for targeted gene delivery in biomedical research and therapeutic applications.
PackGene offers nearly 100 serotypes for our packaging service to assist your research work.
Additionally, the development of AAV mutant serotypes with more tissue specificity and stronger infectivity is crucial for innovation in AAV-mediated gene delivery. PackGene provides comprehensive AAV serotype engineeringg services to offer you a one-stop solution.
However, despite the tissue tropism of wild-type AAVs to some extent, the infection of non-target tissues cannot be completely avoided. In such cases, combining tissue- or cell-specific promoters with serotypes can greatly enhance AAV specificity. PackGene offers various tissue-specific promoters, such as the muscle-specific promoter MHCK7-2 and the liver-specific promoter TBG669. Our piVector Design embed in our online ordering system offers various promoters including universal and tissue specific promoters. You may easily build your vector into our AAV backbones that have been rigorously verified for effective viral packaging.
What features does AAV have comparing to other viral vectors?
AAV vectors stand out for their safety, low immunogenicity, ability to transduce non-dividing cells, and potential for long-term gene expression without integrating into the host genome. These features make them particularly attractive for gene therapy applications targeting diseases where long-term expression and safety are paramount.
However, the limited packaging capacity is a constraint when delivering larger genes. In contrast, vectors like adenovirus and HSV can carry larger genetic payloads but come with higher immunogenicity and safety concerns. Lentiviral and retroviral vectors offer stable, long-term expression through genome integration but carry risks associated with insertional mutagenesis.
By leveraging the unique advantages of AAV, such as tissue-specific targeting through various serotypes and a favorable safety profile, therapies can be designed for a range of genetic disorders with minimized risks. These characteristics contribute to the growing preference for AAV vectors in both research and clinical gene therapy programs.
What are the general considerations when designing AAV iexperiment?
Serotype selection: If you are unsure which AAV serotype is most suitable for your experiments, we advise that you test the infection rates of 3 or more different serotypes in your experimental system with our rAAV fluorescent reporter constructs.
Gradient dilution infection: The level of transgene expression driven by rAAV may vary substantially across different genes. We therefore recommend that you perform 3-4 AAV gradient dose injections to determine the ideal gene expression level for each rAAV before performing any formal experiments.
Experimental control: We advise the use of a GFP positive control vector of the same serotype and promoter as your experimental vector.

AAV Packaging Services
Ranging from pilot to industrial-scale AAV packaging for both in vitro and in vivo studies.
READ MORE

Off the Shelf AAV Products
We offer a wide catalogue of pre-made AAVs for a multitude of research needs.
READ MORE
