Adeno-associated virus (AAV) is a non-pathogenic single-stranded DNA virus. Several features of AAVs position them as an exceptional research tool as well as an attractive candidate for genetic payload delivery in Gene and Cell Therapies. Notable features include: (1) AAV are not currently known to cause any disease, (2) AAV infection results in a very mild immune response, (3) AAV are capable of infecting both dividing and non-dividing cells, and (4) recombinant AAV (rAAV) are capable of driving prolonged expression of a gene of interest (GOI) without integrating the GOI into the host genome. In addition, differences in the capsid structure of various AAV serotypes bias infection rates across host cell-types and therefore provide a mechanism for tissue or cell-type infection specificity.
PackGene provides superior quality AAV packaging services to support your AAV-based programs. We have developed a series of proprietary technologies that greatly improve AAV production outcomes including titer, purity, potency, and consistency.
We offer multiple serotypes to meet your research needs, and we strive to achieve rapid turnaround and affordable prices while maintaining the highest quality. We are committed to delivering AAVs at guaranteed quantities and within the quoted project lead time. In a case where our quantity and efficiency guarantee is not met, we will refund 5% of the total order cost as an account credit that may be applied to your next order.
Fast Turnaround
12-15 business days for AAV 5E+13GC
Guaranteed Titer
≥1E+13GC/mL (qPCR genome copies/ml)
High Purity
High Yield
up to 1E+16 GC
Low Endotoxin
<10EU/ml, suitable for in vivo experiments
Low Empty Shell
<30%
Multiple Serotypes
70+ different serotype options available
Extensive Experience
Successfully delivered over 10,000 custom AAV projects
Experienced Technical Support
PhD-level team with years of AAV experience
*The indicated titers are guaranteed except when the insert exceeds the packaging capacity (4.7 kb) or if you choose to provide us with your own modified rep/cap plasmid or helper plasmid.

If you would like to use any AAV serotypes that are currently under patent, and your application is for commercial use, we advise that you contact the patent owner to obtain authorization beforehand.
| AAV Packaging Serotypes | Guaranteed Yield (GC)* | Lead Time (Business Days) |
| Normal-yield AAV Serotypes | 2E+12 GC | 12-15 Days |
| 5E+12 GC | ||
| 1E+13 GC | ||
| 2E+13 GC | ||
| 5E+13 GC | ||
| 1E+14 GC | ||
| 2E+14 GC | 18-24 Days | |
| 5E+14 GC | ||
| 1E+15 GC | 30-45 Days | |
| 2E+15 GC | ||
| Low-yield AAV Serotypes (AAV4, 6, etc.) | 2E+12 GC | 12-15 Days |
| 5E+12 GC | ||
| 1E+13 GC | ||
| 2E+13 GC | ||
| 5E+13 GC | ||
| 1E+14 GC | 18-24 Days | |
| 2E+14 GC | ||
| 4E+14 GC |

- GC = Genome copies.
- For these extremely low-yield AAV serotypes without production data, we are not able to guarantee the final yield or titer specified here.
Storage Requirements
- Store the virus at -80°C, and place it on ice during operation.
- Calculate your expected usage in advance and PackGene will aliquot your virus according to your pre-determined requirements. This can help avoid unnecessary thawing and re-freezing after receiving your AAVs since freeze thaw cycles influence virus viability. If aliquoting is required, it is recommended to use PCR tubes with siliconized inner walls, or special virus preservation tubes with low protein binding rates.
- Thaw your virus aliquots in an ice bath immediately before use.
- Dilute with PBS or PBS / 0.001% F-68 if needed.
A variety of AAV-based QC assays have been developed by PackGene’s experienced QC team. QC tests are aimed at verification of the identity, purity, and potency of AAV viral particles for both in vitro and in vivo studies. AAV genome copies are quantified via SYBR qPCR with ATCC’s Reference AAV for titer calibration. Purity is determined by Coomassie-Blue staining.
We guarantee the endotoxin level of the AAV particles lower than 10 EU/ml. We also offer additional QC tests including ddPCR , TEM, TCID50 tittering and other QC services. Please check AAV Analytical Services to learn more.
| Category | QC Assays | QC Standard |
| Identity | Identity – GOI Sequence | Additional QC |
| Purity | SDS-PAGE Coomassie Blue Staining | Free QC |
| TEM | Additional QC | |
| AUC | Additional QC | |
| Potency & Content | qPCR | Free QC |
| ddPCR | Additional QC | |
| TCID50 | Additional QC | |
| Capsid Titer-ELISA | Additional QC | |
| Impurity | Endotoxin Test | Free QC |
| Mycoplasma Detection | Additional QC | |
| Sterility Test | Additional QC | |
| Residual Plasmid Test | Additional QC |
Standard QC
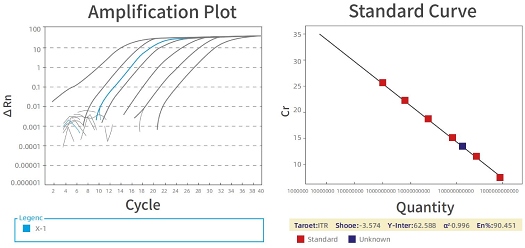
Note: ATCC VR-1816™ was used as the standards for AAV qPCR titering.
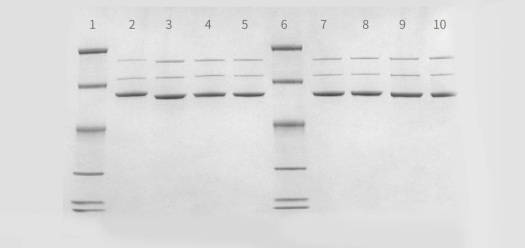
Legend: Lanes 2-5 and 7-1: AAV samples produced at PackGene. Lane 1,6: Marker
Other Available Analytical Tests
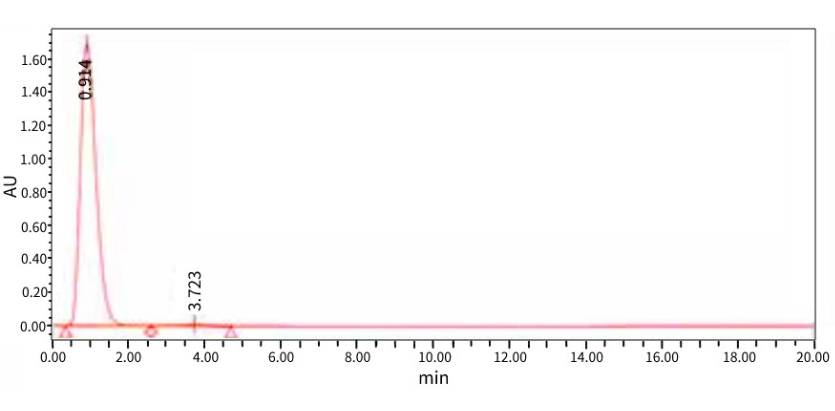
AAV2-EGFP Sample produced at PackGene. The purity as analyzed by HPLC was 99%.
AAV CRISPR/Cas9 Gene Editing: A New Horizon in Huntington’s Disease Treatment
AAV Packaging Protocol
Nanodecoys for Antiviral Applications Against SARS-CoV-2 and Other Viruses

AAV Analytical Services
We are one of the few vendors that can do ALL in-house.
READ MORE

Off-the-Shelf AAV Products
We offer a wide catalogue of pre-made AAVs for a multitude of research needs.
READ MORE
